0897
Thyroxine Treatment in Preterms with Grade III/IV Hemorrhage and Microstructural White Matter Assessment with Diffusion Tensor Imaging: A Pilot Study1Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Radiology, Children's Hospital of Pittsburgh UPMC, Pittsburgh, PA, United States, 3Pediatrics (Neonatology), Albert Einstein College of Medicine, New York, NY, United States, 4Neuroscience, Albert Einstein College of Medicine, New York, NY, United States
Synopsis
Intraventricular hemorrhage (IVH) in preterm infants is a complication that leads to neurodevelopmental delay and neurologic disorders. In this study we used diffusion tensor imaging (DTI) to analyze the myelination and structural integrity of white matter in preterms with and without T4 treatment using a manual uniform region of interest based analysis and semi-automated method of tractography. This small cohort pilot study of preterm subjects with Grade III-IV IVH demonstrated that thyroxine therapy may result in improved microstructural changes in certain tracts, and DTI maybe able to serve as neuroimaging biomarker for treatment efficacy in relation to thyroxine intervention.
Introduction
Intraventricular hemorrhage (IVH) in preterm infants is a complication that leads to neurodevelopmental delay and a constellation of neurologic disorders. The onset of IVH, at 22-32 weeks gestational age (GA), coincides with the maturation phase of oligodendrocyte progenitor cells (OPCs) – precursors to oligodendrocytes that myelinate axons – causing degeneration and reduced proliferation1. Our observations in preterm infants and rabbit pups have shown that OPC growth depression is related to suppressed thyroid hormone signaling and subsequent hypothyroid state. Treatment with thyroxine (T4) in rabbit pups with IVH promoted OPC proliferation, maturation, and myelination with restored neurological function2. In this study we use diffusion tensor imaging (DTI) to analyze the myelination and structural integrity of white matter in preterms with and without T4 treatment. We also validated our method of DTI measurements in a separate cohort of preterm neonates with and without IVH, knowing that IVH can severely effect brain anatomy which can lead to challenges in consistent quantitative methods to analyze DTI data.
Methods and Materials
As part of a pilot study, approved by internal review board, 14 preterm neonates with severe IVH (Grade III-IV) were prospectively recruited and randomly assigned to non-treatment (N=7, GA=25.2±1.9weeks) or T4 treatment (N=7, GA=24.3±1.5weeks) groups. The subjects in the treatment group, within 7 days after birth, received 8mcg/kg of T4 daily for 6 weeks. All subjects were scanned on 3T Achieva (Philips) with the following parameters: FOV=256mm, voxel size=2.0mm(isotropic), TE/TR=70ms/6960ms, B=800s/mm2. We also scanned two additional prospectively recruited cohorts of preterms for comparison and validation of our methods at a separate site (n=105 with Grade 0, I, and II IVH and n=25 preterms with Grade III-IV) in preparation for a multi-center neuroimaging study to study the efficacy of thyroxine groups.
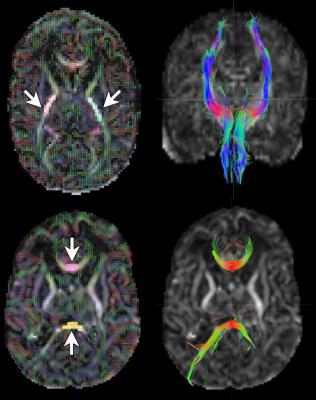
Anaylsis to examine differences between T4 and non-treatment groups was carried out using (1) fully automated Tract Based Spatial Statistics (FSL3); (2) manual region of interest (ROI) based analysis using standardized ROIs to measure fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) inside a uniformed volume of corticospinal tract (level of internal capsule), genu, and splenium tracks; (3) a tract based analysis using DSI studio4 to track CST, splenium, and genu and calculate the mean FA, AD, and RD of as shown in Figure 1. The difference between manual and tractography methods is that the former measures a uniform cross section of a fascicle while the latter quantitate metrics over the entire bundle. Due to the sample size, Kruskal-Wallis (KW) non-parametric rank test was used to compare differences between the two groups.
Results
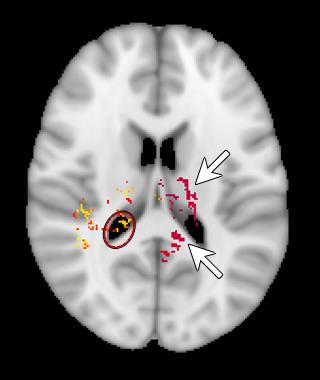
The results from the TBSS analysis are presented in Figure 2. There were regions where T4 group had higher FA than non-treatment group (p<0.05), but no regions where non-treatment groups exhibited higher FA. However, the ventriculomegaly in high grade IVH (grade III-IV), proved challenging to template based registration methods such as TBSS. The regions bordering the ventricles are especially vulnerable to this type of error resulting in stray pixels that do not correspond to any anatomically relevant white matter regions (Figure 2, red circle).
Non-parametric Kruskal-Wallis (KW) analysis of quantiations from manual RO method (GA at scan as covariate) showed significantly higher AD (p=0.0446) and lower RD (p=0.0285) in splenium of T4 compared to non-treatment group. While the FA comparison of the splenium was not significant (p>0.05) a supporting trend with T4 FA higher than non-treatment group was observed.
The tractography analysis showed a significantly higher average FA (p=0.0446) in T4 than non-treatment group. Our methodology was validated in the larger cohort of preterm subjects with and without IVH.
Discussion
This pilot study demonstrated, in a small cohort of preterm subjects with Grade III-IV, that DTI maybe capable of being a neuroimaging biomarker of treatment efficacy in relation to thyroxine intervention. Standardized automated data-driven methods faced challenges in generating quantitative DTI data caused by mis-registration due to prevalence of ventriculomegaly and blood product in preterm IVH. In addition, Grade IV IVH is typically asymmetric compounding the difficulty to standardize in hypothesis driven approaches (tractrography). Our data suggests that thyroxine therapy may results in improved microstructural changes in the splenium of the corpus callosum which is one of the earliest structures to develop pre-myelinated elements during the last trimester of gestation.
Conclusion
Thyroxine therapy in preterms with IVH results in improved microstructural changes in the splenium of the corpus callosum using a semi-automated tractography approach. This study provides the technical rationale for a planned larger Phase II/III thyroxine neuroprotective efficacy study in preterm with IVH using DTI as an intermediate neuroimaging biomarker of treatment response.
Acknowledgements
No acknowledgement found.References
[1] Ballabh, P., 2010. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatric research, 67(1), pp.1-8.
[2] Vose, L.R., Vinukonda, G., Jo, S., Miry, O., Diamond, D., Korumilli, R., Arshad, A., Zia, M.T., Hu, F., Kayton, R.J. and La Gamma, E.F., 2013. Treatment with thyroxine restores myelination and clinical recovery after intraventricular hemorrhage. The Journal of Neuroscience, 33(44), pp.17232-17246.
[3] Jenkinson, M., Bannister, P., Brady, M. and Smith, S., 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17(2), pp.825-841.
[4] Yeh, F.C., Wedeen, V.J. and Tseng, W.Y.I., 2010. Generalized-sampling imaging. IEEE transactions on medical imaging, 29(9), pp.1626-1635.
Figures