0894
Micro- and macro-structural development of the cortex in preterm infants1Centre for the Developing Brain, Division of Imaging Sciences & Biomedical Engineering, King's College London, London, United Kingdom, 2Centre for Medical Image Computing, Department of Computer Science, University College London, London, United Kingdom, 3Department of Neuroimaging, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom, 4Biomedical Image Analysis Group, Department of Computing, Imperial College London, London, United Kingdom
Synopsis
We assessed micro and macrostructural cortical development in preterm infants using cortical curvature and thickness measures and neurite orientation and density imaging (NODDI). We studied two independent datasets of 64 and 42 neonates, and characterised the association between cortical features (median FA, MD, NDI, ODI, mean curvature and cortical thickness) and post-menstrual age (PMA) at MRI and gestational age at birth (degree of prematurity). We found a distinct pattern of development between the term (>37 weeks PMA) and preterm period (<37 weeks PMA), and different growth patterns between somatosensory and fronto-temporal areas.
Introduction
The third trimester of pregnancy is associated with rapid cortical development including cortical gyrification and dendritic arborisation. In infants born preterm these critical processes occur ex-utero and cortical development may be disrupted following preterm birth. Advanced MRI approaches specifically adapted to this population enable gyrification to be characterised using cortical curvature measures and cortical microstructural development to be assessed using multi-compartment models of diffusion MRI (dMRI). In this work we used multi-shell, high angular resolution MRI acquisitions enabling the use of neurite orientation and density imaging (NODDI)[1] to obtain microstructural features including neurite density index (NDI) and orientation dispersion index (ODI). These measures provide complementary information to diffusion tensor (DT) features such as fractional anisotropy (FA) and mean diffusivity (MD). We acquired 64 infants spanning a wide post-menstrual age (PMA) range between 25 and 45 weeks (Dataset 1) to assess cortical development during this critical period. In addition, we studied a cross-sectional dataset of 42 preterm infants at term equivalent age (39–47 weeks PMA) acquired at a different site (Dataset 2) enabling us investigate in detail cortical micro and macrostructure at around the time of normal birth.
Methods
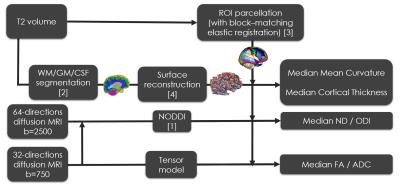
Acquisition: Dataset 1 included 64 infants (8 infants were scanned twice, resulting in a sample size of 72 datasets) born at 24-41 (median 33) weeks gestational age (GA) and imaged between 25-45 (median 36) weeks PMA at St. Thomas’ Hospital. Dataset 2 included 42 infants born at 24-33 (median 30) weeks PMA and imaged between 38-47 (median 41) weeks PMA at Queen Charlotte’s & Chelsea Hospital. T2-weighted anatomical volumes and dMRI including 2 shells of 64/32 non-collinear directions at b=2500/750 s/mm2, respectively were acquired in each dataset, with TR=9000ms, TE=62/49ms and voxel-size 2mm-isotropic.
Pre-processing (Figure 1): T2 images of each subject were segmented into white and grey matter (WM/GM) using an algorithm specifically adapted to neonates[2] and parcelled in 72 ROIs by non-linear registration of a version of the AAL atlas specifically adapted to neonates[3]. In addition, the cortical surface of each subject was reconstructed[4], and measures of mean curvature and cortical thickness obtained for each surface vertex. Diffusion volumes were unwrapped and corrected for eddy currents using FSLv5.0 tools (‘topup’/‘eddy’). Fitting of NODDI and DT models was performed for each subject and median values of FA, ADC, NDI, ODI mean curvature and cortical thickness were obtained in GM tissue and in each cortical ROI.
Statistics: the association between cortical microstructure and PMA at MRI and GA at birth was assessed by Spearman’s correlations regressing out (independently for each dataset) sex, birthweight below the 10th centile and GA at birth (for association with PMA at MRI) and PMA at MRI (for association with GA at birth) covariates. ROI-wise correlations were corrected by False Discovery Rate (FDR) at 5%[5].
Results
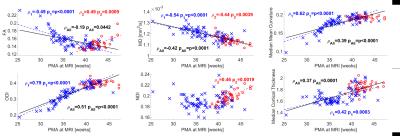
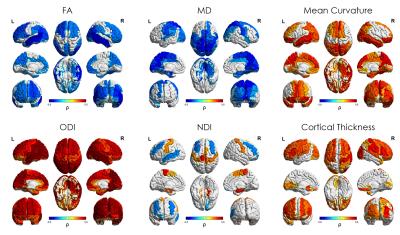
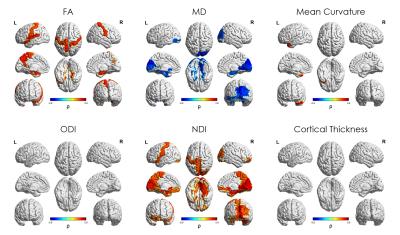
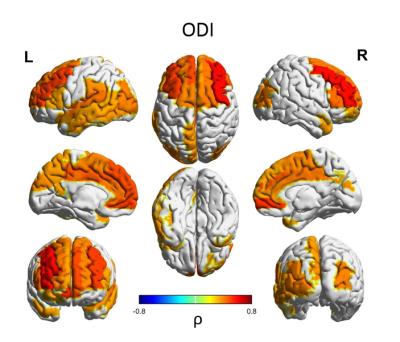
Median values across the whole cortical ribbon showed a pattern of association with PMA at scan coherent between independent datasets but distinct between the term (>37 weeks PMA) and preterm period (<37 weeks PMA)(Figure 2). Likewise, regional correlations showed an association with age at scan, which was distinct between the term (Figure 3) and preterm period (Figure 4), with different growth patterns between somatosensory and fronto-temporal areas. GA at birth was highly correlated with cortical ODI (Figure 5).
Discussion and conclusion
Microstructural features of both datasets showed a complex but coherent pattern of association with neural development. FA values decreased and ODI values increased during the period before the time of normal birth which is consistent with the rapid neuronal arborisation during early development [6,7]. We were also able to characterise an increase in cortical FA after term equivalent age (37 weeks PMA), coherent with a plateau in ODI and an increase in NDI, presumably related to an increase in cellular density during this period. In addition, we observed different regional patterns of maturation by assessing ROI-wise association with PMA at MRI at term equivalent age: NDI clearly showed a positive correlation in somatosensory areas, but a negative association in fronto-temporal, in line with FA, ODI and mean curvature regional patterns. These results are coherent with regional maturational changes in the developing brain, where somatosensory areas develop first and fronto-temporal areas show a more protracted pattern of development.
Importantly, prematurity had a strong effect on cortical ODI, which suggests a maturational delay, particularly in fronto-temporal areas that have a critical role in cognitive function. These results represent a highly detailed characterisation of cortical microstructural features during development, which are consistent between 2 independent datasets, and highlight the potential of this approach to investigate alterations in cortical development.
Acknowledgements
This work was supported by the Medical Research Council (UK) [grant numbers MR/K006355/1 and MR/LO11530/1], and the Department of Health through an NIHR Comprehensive Biomedical Research Centre Award (to Guy’s and St. Thomas’ National Health Service (NHS) Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust).References
1. Zhang, H., T. Schneider, C.A. Wheeler-Kingshott, and D.C. Alexander, NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage, 2012. 61(4): p. 1000-16.
2. Makropoulos, A., I.S. Gousias, C. Ledig, P. Aljabar, A. Serag, J.V. Hajnal, A.D. Edwards, S.J. Counsell, and D. Rueckert, Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans Med Imaging, 2014. 33(9): p. 1818-31.
3. Shi, F., P.T. Yap, G. Wu, H. Jia, J.H. Gilmore, W. Lin, and D. Shen, Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One, 2011. 6(4): p. e18746.
4. Makropoulos, A., P. Aljabar, R. Wright, B. Huning, N. Merchant, T. Arichi, N. Tusor, J.V. Hajnal, A.D. Edwards, S.J. Counsell, and D. Rueckert, Regional growth and atlasing of the developing human brain. Neuroimage, 2016. 125: p. 456-78.
5. Benjamini, Y. and D. Yekutieli, The control of the false discovery rate in multiple testing under dependency. Annals of Statistics, 2001. 29(4): p. 1165-1188.
6. Ball, G., L. Srinivasan, P. Aljabar, S.J. Counsell, G. Durighel, J.V. Hajnal, M.A. Rutherford, and A.D. Edwards, Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci U S A, 2013. 110(23): p. 9541-6.
7. Eaton-Rosen, Z., A. Melbourne, E. Orasanu, M.J. Cardoso, M. Modat, A. Bainbridge, G.S. Kendall, N.J. Robertson, N. Marlow, and S. Ourselin, Longitudinal measurement of the developing grey matter in preterm subjects using multi-modal MRI. Neuroimage, 2015. 111: p. 580-9.
Figures