0892
Fixel-based morphometry detects alterations in specific fibres in association with preterm birth: a proof-of-concept study.1MRC Centre for Reproductive Health, University of Edinburgh, edinburgh, United Kingdom, 2Centre for Clinical Brain Sciences, University of Edinburgh, edinburgh, United Kingdom, 3Clinical Research Imaging Centre, University of Edinburgh, edinburgh, United Kingdom
Synopsis
Preterm birth is closely associated with diffuse white matter injury which contributes to long term neurocognitive impairment among survivors. Fixel-based analysis (FBA) is the study of specific fibre populations within a voxel; it provides measures of fibre bundle morphology by combining information about fibre density with structure. In this work, we applied FBA to neonatal dMRI data and provide proof-of-concept that fibre density and fibre bundle cross section may be useful measures for evaluating alterations to brain development associated with preterm birth.
PURPOSE
Fixel-based analysis (FBA) is a powerful tool for white-matter structural analysis1 that has recently been developed to evaluate white matter morphology in adults2. It provides measures of Fibre Density (FD), Fibre-Bundle Cross-Section (FC), and the combination of both, called Fibre Density and Cross-Section (FDC). In this work, we compare FD, FC and FDC between preterm infants at term equivalent age and healthy term controls, and we show that these parameters may be useful markers of brain development.METHODS
Participants: 10 healthy infants born at full term (mean PMA at birth 39+2 weeks, range 38+4–40+3 weeks) underwent brain MRI at mean age 41+2 weeks; MRI data were also acquired from 10 preterm infants (mean PMA at birth 28+1 weeks, range 26+2–31+3 weeks) at term equivalent age (mean PMA 41 weeks, range 39+1–42+5 weeks). Infants were examined in natural sleep with pulse oximetry, temperature and electrocardiography data monitoring. Ear protection was used for each infant, comprising earplugs placed in the external ear and neonatal earmuffs (MiniMuffs, Natus Medical Inc., CA).
MRI acquisition: A Siemens MAGNETOM Verio 3T MRI clinical scanner was used to acquire: 3D T1-weighted (T1w) MPRAGE data with voxel size = 1×1×1mm3; diffusion MRI (dMRI) data using a protocol consisting of 11 T2- and 64 diffusion-weighted (b = 750s/mm2) single-shot, spin-echo, EPI volumes with 2mm3 isotropic voxels.
Processing: sMRI: Brain masks were computed by removing non-brain tissues and skull using ALFA3 and were corrected for bias field4. dMRI: The first analysis step was to denoise the images5, 6 followed by up-sampling by a factor of 2. After this, eddy current correction was performed7. Then the mask was propagated from the T1w volume using non rigid registration8, 9. Each dMRI dataset was corrected for bias field distortions4, by first estimating a correction field from the B0 image, then applying the field to correct all volumes. To correct for EPI distortions, the T1w volume was co-registered to the B0 volume, then the B0 volume was non-rigidly registered to the T1w volume10, but restricting the deformation direction to only the phase encoding direction11; afterwards, the computed transformation was applied to the rest of the dMRI volumes. Global intensity normalisation across subjects was perform by dividing all volumes by the median b=0s/mm2 intensity within the white matter12, using the white matter mask of the ENA3313. The average response function was used to calculate all the fibre orientation distributions (FODs)12. Spatial normalisation: Spatial correspondence was obtained by registering all FOD images to a symmetrical study-specific FOD template2, 14, 15. Fixel-based analysis: We compared the FD, FC, and FDC in all white matter fixels across both groups using a general linear model. Connectivity-based smoothing and statistical inference were performed with Connectivity-based Fixel Enhancement using 2 million streamlines and default parameters1. Family-wise error corrected p-values were assigned to each fixel using non-parametric permutation testing with 5000 permutations. To visualize the results, the T2-weighted template of the ENA3313 was non-rigid registered to the FOD template8, 9.
RESULTS
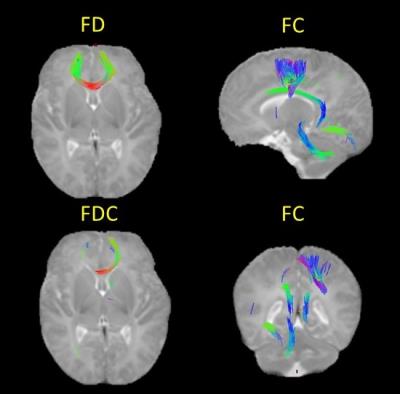
Preterm birth is associated with significant reductions in FC, FD and FDC in major fasciculi, compared with healthy controls born at term (Fig. 1). FD and FDC are reduced in the genu of the corpus callosum; and FC is reduced in the corpus callosum, left cingulum cingulate gyri, the right corticospinal tract, the left inferior longitudinal fasciculi and the left cerebellothalamic tract.DISCUSSION
In this proof of concept study, we found that FBA detects differences in fibre bundle morphology in preterm infants at term equivalent age compared with healthy term controls. The results are consistent with previous studies reporting differences in the major tract microstructure and topology in association with preterm birth16.
The difference in FD in the genu indicates a reduction in the intra-axonal compartment per unit volume of tissue in the preterm group, and the observation that FDC is reduced across most of the genu indicates that this is associated with decreased total intra-axonal volume.
The differences in the rest of the regions are due to the FC, meaning that in these tracts there is a reduced extra-axonal volume, suggesting a narrowing of the diameter of the tract in preterm infants.
CONCLUSION
Fixel-based analysis is a promising approach for studying functionally tractable measures of information carrying capacity inferred from axonal number and tract volume in the newborn brain. Further studies of a larger population are warranted to investigate the anatomic distribution of change in FD, FC, and FDC associated with preterm birth, and the predictive values of neonatal FBA parameters for long term neurocognitive outcome.Acknowledgements
We are grateful to the families who consented to take part in the study and to the nursing and radiography staff at the Clinical Research Imaging Centre, University of Edinburgh (http://www.cric.ed.ac.uk) who participated in scanning the infants. The study was supported by Theirworld, NHS Research Scotland, and NHS Lothian Research and Development. We thank Thorsten Feiweier at Siemens Healthcare for collaborating with dMRI acquisitions (Works-in-Progress Package for Advanced EPI Diffusion Imaging).References
1. Raffelt DA, Smith RE, Ridgway GR, Tournier JD, Vaughan DN, Rose S, et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage. 2015;117:40-55.
2. Raffelt DA, Tournier JD, Smith RE, Vaughan DN, Jackson G, Ridgway GR, et al. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage. 2016.
3. Serag A, Blesa M, Moore EJ, Pataky R, Sparrow SA, Wilkinson AG, et al. Accurate Learning with Few Atlases (ALFA): an algorithm for MRI neonatal brain extraction and comparison with 11 publicly available methods. Scientific Reports. 2016;6:23470.
4. Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE transactions on medical imaging. 2010;29(6):1310-20.
5. Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. NeuroImage.
6. Veraart J, Fieremans E, Novikov DS. Diffusion MRI noise mapping using random matrix theory. Magnetic Resonance in Medicine. 2016;76(5):1582-93.
7. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-90.
8. Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. Medical Imaging, IEEE Transactions on. 1999;18(8):712-21.
9. Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, et al. Fast free-form deformation using graphics processing units. Computer Methods and Programs in Biomedicine. 2010;98(3):278-84.
10. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26-41.
11. Wu M, Chang LC, Walker L, Lemaitre H, Barnett AS, Marenco S, et al. Comparison of EPI Distortion Correction Methods in Diffusion Tensor MRI Using a Novel Framework. Medical image computing and computer-assisted intervention : MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention. 2008;11(Pt 2):321-9.
12. Raffelt D, Tournier JD, Rose S, Ridgway GR, Henderson R, Crozier S, et al. Apparent Fibre Density: a novel measure for the analysis of diffusion-weighted magnetic resonance images. Neuroimage. 2012;59(4):3976-94.
13. Blesa M, Serag A, Wilkinson AG, Anblagan D, Telford EJ, Pataky R, et al. Parcellation of the healthy neonatal brain into 107 regions using atlas propagation through intermediate time points in childhood. Frontiers in Neuroscience. 2016;10.
14. Raffelt D, Tournier JD, Fripp J, Crozier S, Connelly A, Salvado O. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage. 2011;56(3):1171-80.
15. Raffelt D, Tournier JD, Crozier S, Connelly A, Salvado O. Reorientation of fiber orientation distributions using apodized point spread functions. Magn Reson Med. 2012;67(3):844-55.
16. Anblagan D, Bastin ME, Sparrow S, Piyasena C, Pataky R, Moore EJ, et al. Tract shape modeling detects changes associated with preterm birth and neuroprotective treatment effects. NeuroImage: Clinical. 2015;8(0):51-8.