0884
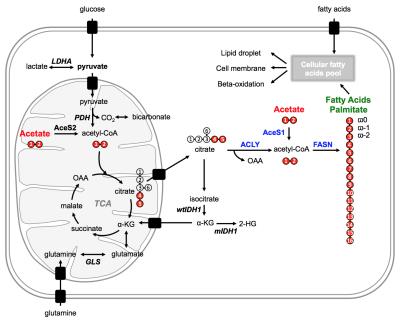
Acetate metabolism towards fatty acids is down-regulated in IDH1 mutant glioma as shown by 13C MRS1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States
Synopsis
IDH1 is the most prevalent driver mutation in lower-grade glioma and upgraded glioblastoma and is associated with additional metabolic reprogramming. Here, we investigated fatty acid biosynthesis and the role of acetate, which was recently recognized as a major fuel in primary glioblastoma. Labeling cells harboring either wild-type or mutant IDH1 with [1,2-13C]-acetate, we found a decrease in flux towards fatty acids in mutant IDH1 cells although the total fatty acid pools remained unchanged. Associated with cell biological assays, these results point to alternate sources for maintenance of fatty acid levels in IDH1 mutant cells.
Introduction
The mutation in the cytosolic form of isocitrate dehydrogenase (IDH1) is the most prevalent mutation in lower-grade glioma (LGG, grade II/III) and upgraded glioblastoma, and is now recognized as a “driver mutation” required for the initiation of LGG [1]. Beyond production of 2-hydroxyglutarate (2-HG), studies show that the IDH1 mutation is associated with several MRS-detectable metabolic changes [2,3]. Recently, acetate has been shown as an important source of nutrients in glioblastoma and brain metastases [4,5] but the potential role of acetate in IDH1 mutant (IDH1mut) glioma remains to be elucidated. Here, we investigated using 1H and 13C MRS the role of acetate in fatty acid biosynthesis in a well-characterized genetically-engineered model of IDH1mut glioma.Material and methods
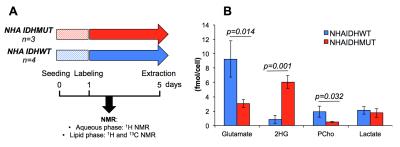
Cell culture An immortalized normal human astrocyte-based cell line (NHA) was transduced to express mutant IDH1 (NHAIDHmut) or wild-type IDH1 (NHAIDHwt). Cells were grown as monolayers in DMEM supplemented with 10% FBS, 100units/mL penicillin and 100μg/mL streptomycine. Cells were labeled with 5mM [1,2-13C]-acetate for 96 hours and then extracted (~6x107, n=3/4 NHAIDHmut/NHAIDHwt) using the dual-phase extraction method as previously described [6] (Fig.2A).
Cell extraction Following phase separation, the aqueous phase was lyophilized and resuspended in 400μL of deuterium oxide, and the lipid phase was resuspended in 400μL of deuterated chloroform. 43mM sodium 3-(trimethylsilyl)propionate-2,2,3,3-d4 (TSP) placed in a coaxial insert was used as an external chemical shift and quantification reference.
MRS data acquisition and analysis Aqueous and lipid phase spectra were recorded using a 500MHz Bruker Avance spectrometer equipped with a triple resonance cryoprobe. 1H spectra were acquired using a 90deg flip angle and 3s repetition time (number of transient (NT)=192) and proton-decoupled 13C spectra were acquired using a 30deg flip angle and 3s repetition time (NT=2400). In addition, fully relaxed 1H and 13C spectra (90deg/TR=60s) were recorded and served to determine correction factors for saturation and NOE (13C acquisitions only). Prior to Fourier transformation, a line broadening of 0.3Hz and 1Hz were applied to 1H and 13C FIDs respectively. All 1H and 13C spectra were quantified by peak integration using MestRenova (Mestrelab). Peak integrals were then corrected using correction factors and normalized to cell number and to TSP external reference.
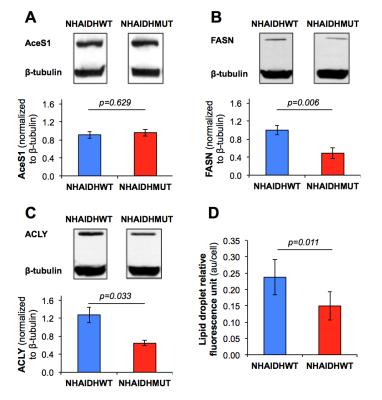
Immunoblotting and spectrophotometric assay Acetyl-CoA synthase (AceS1), fatty acid synthase (FASN) and ATP citrate lyase (ACLY) expressions were measured by immunoblotting and lipid droplet levels were determined using fluorescence assay (Cayman).
Statistical analysis All results are expressed as mean±s.d. An unpaired two-tailed Student’s t-test with equal variance was used, with a p-value<0.05 considered as significant.
Results & Discussion
After cellular uptake, acetate is converted to acetyl-CoA, a key metabolic intermediate that fuels the TCA cycle and is an essential building block for the biosynthesis of fatty acids (Fig.1).
Aqueous phase As illustrated in Fig.2B, addition of 5mM [1,2-13C]-acetate to cellular growth media did not alter the metabolic profile of NHAIDHmut. Beyond 2-HG production, NHAIDHmut cells significantly down-regulate phosphocholine and glutamate and have a reduced lactate level compared to NHAIDHwt as previously reported [2].
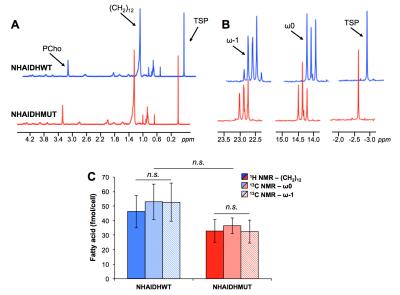
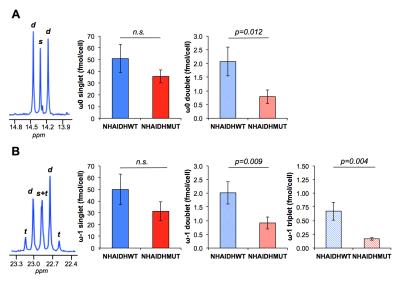
Lipid phase 1H data from lipid phases showed no difference in total fatty acid levels (C16:0, palmitic acid at 1.2ppm) between NHAIDHwt and NHAIDHmut cells (Fig.3A/3C). In the 13C spectra, quantification of the ω0 pool (singlet+doublet) at 14.3ppm as well as the ω-1 pool (singlet+doublet+triplet) at 23ppm, also representing total fatty acids, confirmed the 1H data (Fig.3B/3C). However, signal from the ω0 doublet, as well as the ω-1 doublet and triplet signals, which are associated with flux of 13C-labeled acetate towards fatty acids (Fig.1), showed a significant decrease (p<0.012) in NHAIDHmut cells (Fig.4). These results suggest that acetate metabolism towards fatty acid synthesis is significantly decrease in NHAIDHmut as compared to NHAIDHwt cells.
Cell biological assays This decrease in acetate metabolism was associated with a reduced expression (p<0.033) in FASN and ACLY, two enzymes involved in fatty acid synthesis from acetyl-CoA, whereas expression of AceS1, the cytosolic enzyme converting acetate to acetyl-CoA, was not altered (Fig.5A/5B/5C). Finally, a significant drop in lipid droplet accumulation (p=0.011) was observed in NHAIDHmut cells by spectrophotometric assay (Fig.5D). Together, these results suggest that metabolism of acetate towards fatty acids is significantly decreased (~60%) in NHAIDHmut as compared to NHAIDHwt cells although acetate conversion to acetyl-CoA is unchanged. This points to alternate sources for fatty acids (glucose, glutamine, uptake from serum) in IDH1mut cells and suggests that fatty acids are preferentially directed towards cell membrane assembly.
Conclusion
We demonstrate here the feasibility of using [1,2-13C]-acetate to study fatty acid metabolism in IDH1mut glioma. Our results show decreased fatty acid synthesis in IDH1mut cells although fatty acid steady state level remains unchanged.Acknowledgements
Work supported by NIH R01CA172845, NIH R21CA201453, NIH R01CA1972254, UCSF Brain Tumor Center Loglio Collective, and center grant P41EB013598References
[1] Cohen AL et al., Current neurology and neuroscience reports 2013 [2] Izquierdo-Garcia JL et al., PloS one 2015 [3] Viswanath P et al., Oncotarget 2016 [4] Mashimo T et al., Cell 2014 [5] Comerford SA et al., Cell 2014 [6] Ronen SM et al., British journal of cancer 2001Figures