0881
Cervical spinal cord and brain MRS alterations in normal appearing white matter of multiple sclerosis (MS) patients at 3T1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland, 2Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 3Swiss Paraplegic Centre, Nottwil, Switzerland, 4Institute of Neuroradiology, University Hospital Zurich, Zurich, Switzerland, 5Institute of Physics, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 6Department of Psychiatry, Psychotherapy and Psychosomatics Hospital of Psychiatry, University of Zurich, Zurich, Switzerland, 7Neuroimmunology and Multiple Sclerosis Research, Department of Neurology, University Hospital and University Zurich, Zurich, Switzerland
Synopsis
This investigation explores alterations of both cervical spinal cord and brain metabolite levels in normal appearing tissue in patients suffering from multiple sclerosis (MS). In the brain, a 2 dimensional J-resolved spectroscopy sequence and 2D prior-knowledge fitting is used and the metabolite cycling technique is applied to the cervical spinal cord at 3T. Relapsing-remitting MS (RRMS) and secondary progressive MS (SPMS) patients are included and alterations between brain and spinal cord metabolites are addressed.
Purpose:
Multiple sclerosis(MS) is an immune mediated, demyelinating and neurodegenerative disease of the brain(B) and spinal cord(SC)1. Anatomical MRI has limited value in predicting clinical patient status or the future course of the disease. Magnetic resonance spectroscopy(MRS) provides the means of accessing biochemical information from the neural tissue thus holding potential for better characterization of the disease and clinical decision-making. Metabolic alterations in N‐acetyl‐aspartate(NAA), creatine+phosphocreatine(Cr), choline-containing compounds(Cho), glutamate+glutamine(Glu+Gln=Glx) and myo-inositol(mI) have previously been investigated in brain tissue of MS patients2,3,4. In the SC, reduction of the NAA level has been reported5,6. In the present study, for the first time, we examine metabolic alterations in the normal appearing white matter(NAWM) of both brain and SC at level C3/C4 in different subgroups of MS.Data and Methods:
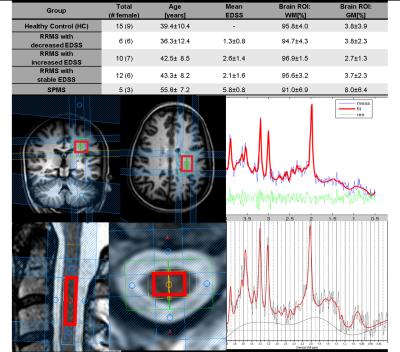
Spectra were measured in 15 healthy controls(HC), 5 secondary progressive MS(SPMS) patients and 28 relapsing-remitting MS(RRMS) patients. The RRMS group is subdivided into three groups based on whether the expanded disability status scale (EDSS) has decreased(RRMSd), increased(RRMSi) or been stable(RRMSs) within the last 12 months before the patient underwent the MR examination. Fig.1 shows the demographics of the study population, the voxel localization and an RRMS patient sample spectrum of the brain and the SC.
MRS Brain: The spectroscopic voxel (25x20x20 mm3) was placed in the periventricular NAWM area (Fig.1 middle) based on a T2 and a highly resolved 3D T1 weighted scan(1x1x1mm3). An echo time series protocol (JPRESS) was used to acquire high spectral data at 3T (Achieva,Philips Healthcare,Best,The Netherlands) as previously7 reported[TE=30ms:228ms, step=2ms, TR=1.6s]. JPRESS data were evaluated using ProFit2 including metabolite basis sets of 18 brain metabolites8. The voxel composition of white matter(WM), gray matter(GM) and cerebrospinal fluid(CSF) were calculated using the Statistical Parametric Mapping (SPM; Wellcome Trust Centre for Neuroimaging, London, UK) segmentation routine.
MRS Spinal Cord: The spectroscopic voxel(6x9x35 mm3) was placed in the normal appearing tissue at the spinal level C3/C4(Fig.1 bottom) based on a T2 weighted scan (0.4x0.4x4mm3) and using the metabolite cycling technique10. 512 signal averages were recorded [TE=30ms, TR=2000-2500ms (heart beat triggered)] and an apodization filter after 200ms was applied before quantification with LCModel (Provencher SW, 1993). The neurovascular SENSE coil was used to acquire the brain and spinal spectra and the total scan time was 65 minutes. All statistical analyses were performed using R Version 3.3.1 (R Core Team, 2016). The data were stratified in subgroup analyses of HC versus SPMS versus RRMS and its subgroups. Metabolic concentration ratio values were compared applying a MANOVA and two-tailed independent samples t-tests were used to identify differences post-hoc.
Results:
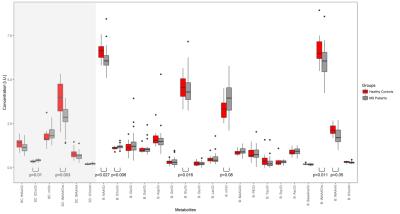
Accurate voxel placement resulted in a very high WM content without significant contamination from GM or CSF. The concentrations of GABA and acetate in the WM brain ROI were too low to be fitted reliably (CRLB>>50). In the SC, tNAA (NAA+NAAG), tCho (GPC and PCh), mI (myo-inositol and glycine) and creatine (Cr) were reliably (CRLB<20) detected(Fig.2). Concentration differences between HC and the patient groups were observed in SC and brain for tNAA/tCho(SC/B:p=[0.003,0.011]) and tCho/Cr(SC/B:p=[0.01,0.006]). Additionally, in brain only, differences were found for tNAA/Cr(p=0.027), mI/Cr(p=0.08), Glu/Cr(p=0.016) and tNAA/mI(p=0.05) ratios. Considering only these metabolite ratios(Fig.3), statistical significant differences (false discovery rate corrected) occur between HC and RRMSi in tNAA/tCho(SC/B:p=[0.003,0.017]) and tCho/Cr(SC/B:p=[0.019,0.007]. HC and RRMSs have a significantly different tNAA/tCho(SC:p=0.003]) and tCho/Cr(SC:p=0.019) ratio. RRMSi and SPMS differ in the brain Glu/Cr ratio(p=0.028) and RRMSi and RRMSs have different brain tCho/Cr ratio(p=0.007). tNAA/tCho of the SC significantly correlates with tNAA/tCho of the brain (p<0.001).Discussion:
Changed concentration values of specific metabolites detected by MRS may be an indicator of an altered metabolism in NAWM of MS patients reflecting inflammation and neurodegeneration or an aggressive course of RRMS. According to previous publications11, highly significant differences between HC and MS patients have been reported for NAA(lower in MS). In our study, we observed additional changes in metabolite ratios such as tCho/Cr or tNAA/tCho in both brain and SC. A number of metabolites including tNAA, Glu, tCho and mI seem to have discriminative power with regard to distinguishing MS subgroups. tNAA/tCho might be a marker of global metabolitic abnormalities in NAWM of MS patients.Conclusion and Outlook:
While further data are being collected, the significance of these changes in NAWM will be verified in a larger cohort of MS patients in longitudinal studies to better understand the predictive value of metabolic profiles for different disease stages or courses of MS. The sensitivity of brain and SC MRS to distinguish clinical subgroups of RRMS with a difference in previous EDSS scores evolvement holds the potential for the use of MRS in therapy monitoring.Acknowledgements
Funding by the Swiss National Science Foundation (Grant Number: 143715) and support by the Clinical Research Priority Project-MS of the University Zurich are gratefully acknowledged.References
1. Compston A et al. The Lancet 2008;372(9648):1502-17 . 2. Siobhan ML et al. Magnetic Resonance Imaging 2000;18(4):455-459. 3. Chard DT et al. Brain 2002;125(10):2342-2352. 4. Srinivasan R et al. Brain 2005;128(5):1016-1025. 5. Kendi AT et al. Neuroradiology 2004;46(9):764-9. 6. Marliani AF et al. Am J Neuroradiol 2010;31:180-4 7. Wyss PO et al. ISMRM Spectroscopy Workshop. Lake Constance, Germany 2016:30. 9. Fuchs A et al., Magnetic Resonance in Medicine 2014;71(2):458-468. 10. Hock A et al., Magnetic Resonacne in Medicine 2013;69(5):1253-60. 11. Sarchielli P et al. Brain 1999;122(3): 513-521.Figures