0879
Reduced GABA levels correlate with cognitive impairment in relapsing-remitting multiple sclerosis1Shandong Medical Imaging Research Institute,Shandong University, Jinan,Shandong, People's Republic of China, 2Philips Healthcare, Shanghai, China, People's Republic of China
Synopsis
To investigate whether cognitive impairment in relapsing-remitting multiple sclerosis (RRMS) is associated with alterations in brain gamma-aminobutyric acid (GABA) levels, 31 RRMS patients and 26 healthy controls underwent 3T 1H MRS and neuropsychologic assessment. GABA levels were quantified in the posterior cingulate cortex (PCC), the medial prefrontal cortex (mPFC) and left hippocampus (LHC). Patients showed reduced GABA+ in PCC and LHC, and reduced GABA+ levels correlated with cognitive function. This study demonstrates that abnormalities of the GABAergic system may be an important neurochemical mechanism of cognitive impairment in RRMS.
Introduction
Cognitive impairment can occur in patients with early phase relapsing-remitting multiple sclerosis (RRMS). Gamma-aminobutyric acid (GABA) is associated with numerous aspects of cognitive function[1], and previous studies have demonstrated that there is a dysfunctional GABAergic neurotransmission in the central nervous system in animal models of MS[2,3]. However, to date there has been a paucity of studies exploring correlation between GABA levels and cognitive function in patients with MS. In this study, therefore, J-difference edited MRS was used to investigate brain GABA levels in patients with RRMS and healthy controls, and their relationship to cognitive function.Methods
31 RRMS patients and 26 age-, gender- and education-matched healthy controls were recruited in this study. All subjects were scanned on a 3 T scanner (Philips “Achieva” TX, Best, The Netherlands) using an eight-channel phased-array head coil for receive. T1-weighted three-dimensional TFE images were used as a localizer. The MEGA-PRESS sequence was performed to detect GABA signal from three brain regions: (1) medial prefrontal cortex (mPFC) (2×3×3 cm3); (2) posterior cingulated cortex (PCC) (3×3×2 cm3); (3) left hippocampus (LHC) (4×2×2 cm3), as shown in Fig. 1. The MEGA-PRESS data were analyzed using 'Gannet' (GABA-MRS Analysis Tool) in Matlab with Gaussian curve fitting to the GABA+ peaks. All subjects were assessed using a set of standardized comprehensive cognitive tests. Information processing speed was assessed using the Symbol-Digit Modalities Test (SDMT). Executive function was measured using the Trail-making test (TMT). Verbal memory was assessed using the Rey Auditory Verbal Learning Test (RAVLT) for immediate and delayed recall. Visuospatial memory was assessed using the Rey-Osterrich complex figure test. Attention was assessed using the Stroop colour word interference test. The raw scores were standardized (z-transformed) based on the mean and SDs of the healthy control subjects. Group comparisons used analysis of covariance (ANCOVA). Between-group GABA levels comparison controlled for GM/(GM+WM) ratios and all between-group neuropsychological comparisons controlled for the influence of education. Correlations between GABA+ levels and neuropsychological scores in patients with RRMS were tested using the Pearson correlation coefficients.Results
Patients showed reduced GABA+ in the PCC (1.047±0.172 IU (patients) vs 1.138±0.128 IU (controls), p=0.03) and LHC (1.184±0.223 IU (patients) vs 1.331±0.263 IU (controls), p=0.024) compared with controls. No statistical difference in GABA+ in the mPFC was seen between groups (p=0.064). Significant lower cognitive scores (RAVLT, SDMT, TMT) were found in patients compared with controls (p<0.05). In patients with RRMS, TMT scores were associated with GABA+ levels in PCC (r= -0.431,p=0.016); significant correlations between RAVLT scores and GABA+ were found in LHC (r=0.372,p=0.039), as shown in Fig. 2; no significant correlations between cognitive scores and GABA+ were found in mPFC.Discussion
The main results of this study are that significantly decreased GABA+ levels were present in the PCC and LHC of patients with RRMS, and GABA+ levels correlated with cognitive scores. This is the first in vivo demonstration of reduced GABA+ concentrations in patients with RRMS, and the first in vivo demonstration of a relationship between GABA levels and cognitive function in patients with RRMS. The results might demonstrate a mechanistic connection between the GABAergic neurotransmission and cognitive function. Reduced GABA levels may be due to loss of inhibitory neurons, dysfunctional GABA synthesis or to dysfunction in enzymes involved in the glutamate/GABA-glutaminecycle. The observed reduced GABA+ levels may reflect deficits of GABA-mediated synaptic transmission, leading to altered inhibitory signaling within cortical circuits, this may ultimately result in the loss or deterioration in cognitive function[4]. Therefore, these results suggest that the neurochemical characteristics of PCC and hippocampus statistically explain part of cognitive impairment in RRMS.Conclusion
This study demonstrates that abnormalities of the GABAergic neurotransmission may be present in the pathogenesis of MS and provides a novel imaging marker for the diagnosis and treatment of cognitive impairment in MS.Acknowledgements
This project was supported by the Shandong Provincial Natural Science Foundation of China (grant nos. BS2015YY003) and Shandong Provincial Medical and Healthy Technology Development Program of China (grant nos. 2015WS0176).References
[1] Michels L, Martin E, Klaver P, et al. Frontal GABA levels change during working memory. PLoS One. 2012;7(4):e31933.
[2] Musgrave T, Tenorio G, Rauw G, et al. Tissue concentration changes of amino acids and biogenic amines in the central nervous system of mice with experimental autoimmune encephalomyelitis (EAE). Neurochem Int. 2011;59(1):28-38.
[3] Rossi S, Muzio L, De Chiara V, et al. Impaired striatal GABA transmission in experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011;25(5):947-56.
[4] Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;723184
Figures
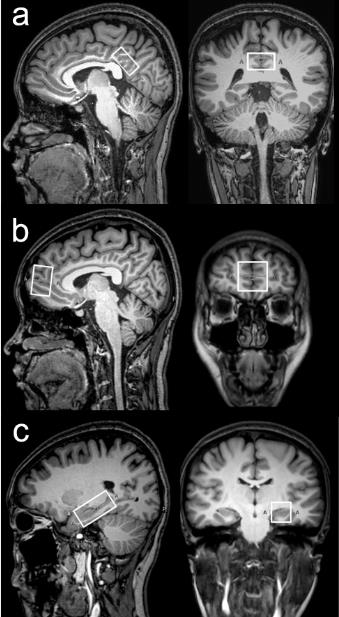
The position of voxels .The (a) , (b) and (c) panels show the position of volumes of interest in PCC, mPFC and LHC on sagittal and coronal T1-weighted TFE images in an RRMS patient.
PCC, posterior cingulate cortex; mPFC, medial prefrontal cortex; LHC,left hippocampus.
