0844
Sleep deprivation affects white matter integrity in cognitively vulnerable individuals1University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Using DTI, we examined the effects of one night of acute total sleep deprivation on fractional anisotropy (FA), an index reflecting the degree of anisotropic water diffusion in brain white matter. Sleep deprivation significantly increases FA in the right superior longitudinal fasciculus (SLF) in individuals who were cognitively vulnerable to sleep loss, while no FA changes were observed in cognitively resistant individuals. Vulnerable subjects also showed lower FA in the right SLF than resistant subjects at baseline before sleep loss, suggesting both trait- and state-dependent interactions between SLF microstructure and cognitive vulnerability to sleep deprivation.
Introduction Insufficient sleep has multiple etiologies and is pervasive in contemporary societies. Millions of people sleep less than 7 hours per night, which is the minimum sleep duration to prevent cumulative deterioration in cognitive performance. Sleep loss increases accident risk and susceptibility to illness, and degrades a broad range of cognitive functions associated with alertness, attention, and memory 1-4. Sleep loss also disrupts multiple brain function 4-6. However, whether and how sleep loss affects brain white matter integrity remain unknown. In this study, we used diffusion tensor imaging (DTI) to examine the effects of one night of acute total sleep deprivation (TSD) on fractional anisotropy (FA), an index reflecting the degree of anisotropic water diffusion in brain white matter.
Methods Fifty-one healthy adults (23 females, mean age = 33.2 ± 8.9 years) participated in a 5-day and 4-night in-laboratory controlled protocol. They were randomized to either a TSD condition (n=39) without sleep on night 2, or a control condition (n=12) with 8-9 hour normal sleep on every night. Using a Siemens 3T Trio scanner, DTI data were acquired on the morning (0700-1000) of days 2 and 3. Participants also completed a 10-minute psychomotor vigilance test (PVT) during each scan and the PVT performance was used to determine their cognitive vulnerability to sleep loss. Imaging data were analyzed using SPM8 and PANDA toolbox.
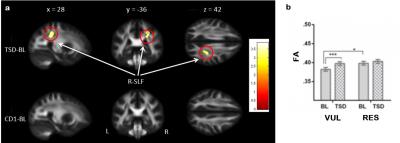
Results The control group showed no differences in FA between the two scans. However, in the TSD group, significant FA increases were found in the right superior longitudinal fasciculus (SLF, Fig.1a), the axonal pathway connecting the frontal and parietal regions, after one night of sleep loss compared to baseline. When the TSD group was median split into individuals who were cognitively vulnerable (n=20) or resistant (n=19) to sleep loss based on the PVT performance changes after TSD, FA increases were only observed in the vulnerable subjects, while no changes were found in the resistant subjects (Fig.1b). Moreover, vulnerable subjects showed significantly lower FA in the right SLF than resistant subjects at baseline before sleep loss (Fig.1b).
Conclusions Higher baseline FA in cognitive resistant individuals is consistent with previous studies 7,8 and supporting the view that differential white matter microstructural properties may contribute to cognitive vulnerability to sleep deprivation. In this study, vulnerable subjects also exhibited increased FA after sleep loss, suggesting both trait- and state-dependent interactions between SLF microstructure and cognitive vulnerability to sleep deprivation.
Acknowledgements
This study is supported in part by NIH grants R01-HL102119, R01-MH107571, R01-NR004281, P30-NS045839, CTRC UL1RR024134, and the PENN IOA Pilot Project.References
1. Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007; 3: 519-528.
2. Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008; 1129: 305-322.
3. Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep 2015; 38: 1161-1183.
4. Goel N, Rao H, Durmer JS, et al. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009; 29: 320-339.
5. Fang Z, Spaeth AM, Ma N, et al. Altered salience network connectivity predicts macronutrient intake after sleep deprivation. Sci Rep. 2015; 5: 8215.
6. Ma N, Dinges DF, Basner M, et al. How acute total sleep loss affects the attending brain: a meta-analysis of neuroimaging studies. Sleep. 2015; 38: 233-240.
7. Rocklage M, Williams V, Pacheco J, et al. White matter differences predict cognitive vulnerability to sleep deprivation. Sleep. 2009; 32:1100-1103.
8. Cui J, Tkachenko O, Gogel H, et al. Microstructure of frontoparietal connections predicts individual resistance to sleep deprivation. Neuroimage 2015; 106: 123-133.
Figures