0842
Diffusion time dependence of kurtosis reveals microstructural changes after neonatal hypoxia-ischemia1Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Radiology, New York University School of Medicine, NY, United States
Synopsis
Apparent diffusion coefficient (ADC) and diffusion kurtosis are both sensitive markers to ischemic brain injury. We investigated the diffusion time (td)-dependency of ADC and kurtosis at nine td’s ranging from 2.5 to 60 ms in a mouse model of neonatal hypoxic-ischemic injury. In the hippocampus, ADCs showed a monotonous decrease with increasing td, whereas kurtosis reached its maximum at td of 5-10 ms and decreased for longer td’s . At the shortest td in this study, we found significant increased kurtosis in the edema region but no significant reduction in diffusivity, suggesting their different sensitivities to microstructural changes after ischemic injury.
Purpose
Diffusion MRI can sensitively detect acute ischemic stroke, but the microstructural basis of the rapid decrease in apparent diffusion coefficient (ADC) within hours after the insult remains elusive. Recent studies suggested that diffusion kurtosis imaging (DKI)1-2 and diffusion time (td)-dependent diffusion MRI can reveal additional information on microstructural organization after ischemic stroke3-7. A combination of these techniques can potentially lead to a more comprehensive characterization of stroke injury. In this study, we hypothesized that kurtosis would have a strong td-dependency, which may reflect microstructural alternations after ischemic stroke. We investigated the behavior of diffusion kurtosis and its change in pathology over a wide range of diffusion times using oscillating and pulsed gradients diffusion encodings in a mouse model of neonatal hypoxic-ischemic (HI) brain injury.Methods
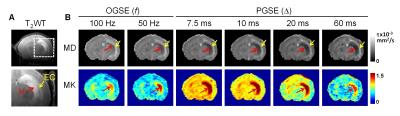
Neonatal C57BL/6 mice (n = 6) at postnatal day 10 were subjected to unilateral HI and imaged at 24 hrs after injury. In vivo MRI were performed on an 11.7 Tesla Bruker MRI system with a 72 mm quadrature volume transmitter and a 10 mm planar surface coil. Diffusion MRI data were obtained using a pulsed gradient spin-echo (PGSE) sequence with δ = 4 ms and Δ = 7.5, 10, 12.5, 15, 20, 40, and 60 ms (td ~ Δ), as well as a cosine-trapezoid oscillating gradient spin-echo (OGSE) sequence with oscillating frequencies (f) of 50 and 100 Hz (td = 1/(4f) = 5 and 2.5 ms as conventionally defined5, see (8) for a more precise relation). Other imaging parameters include: two-shot EPI readout, TE/TR = 56/3000 ms (TE = 65ms for Δ = 60ms), NA=2, 18 diffusion directions, b = 1000 and 2000 s/mm2, in-plane resolution = 0.2x0.2 mm2, 0.8 mm slice-thickness, and scan time of 8 mins for each td. The mean, axial, and radial diffusivity/kurtosis (MD/MK, D// / K//, and D⊥/ K⊥) were calculated at each td.Results
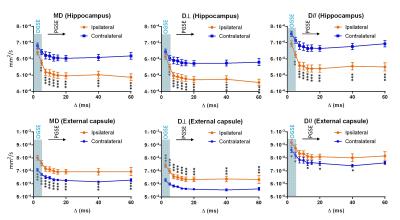
Acute edema was marked by hyperintense T2 signals in the neonatal mice at 24 hrs after HI insult (Fig. 1A). The edema in the hippocampus (H, red arrows) showed reduced MD and elevated MK, whereas the neighboring external capsule (EC, yellow arrows) showed increased MD and no apparent change in MK (Fig. 1B). All diffusivity measurements decreased rapidly with increasing td (up to Δ = 10 ms, Fig. 2) and the rate of ADC decrease slowed down between Δ = 10-60 ms. The contrast between the hippocampal edema and the contralateral side began to show at short td, which were further enhanced at long td, congruent with previous studies6,9. In contrast, the ipsilateral EC exhibited significantly increased diffusivities compared to the contralateral side at all td’s.
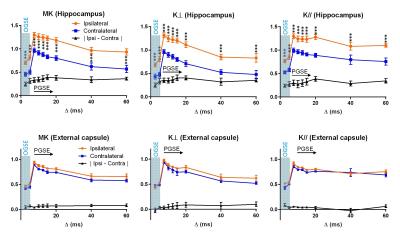
The estimated kurtosis values demonstrated a non-monotonic change with respect to td (Fig. 3) with the peak located near Δ=7.5 ms. Unlike the diffusivity measurements, the hippocampal edema region showed significantly higher kurtosis already at the shortest time points corresponding to the OGSE measurement, with the peak of ipsilateral/contralateral difference around Δ =15 – 20 ms. Spatially, at the short td, only the core of the edema region showed high MK, and the region with high MK gradually expanded as td increased (Fig. 1B). The kurtosis in ipsilateral EC was slightly lower than the contralateral side but the differences were not signficant. In both hippocampus and EC, K⊥ showed more rapid decrease from the peak than K//.
Discussion
While the time-dependent change of water diffusivity in the brain has been investigated before5-7,9,10, the time-dependency of kurtosis remained largely unexplored. Our findings of the non-monotonic change of kurtosis with td agreed with a previous study of kurtosis in the rat cortex11 and simulation studies12. In particular, the initial increase of kurtosis is indicative of freely diffusive molecules gradually reaching the restrictions, in contrast to the monotonically decreasing kurtosis for a medium with spatially varying local diffusivity8. It is interesting that, at the shortest td here, MK clearly marked acute edema in the hippocampus (~ 75% of the maximum contrast) when the diffusivity measurements were still at less than 30% of the maximum contrast. This suggests that the full effects of HI-related microstructural changes on diffusivity measurements become pronounced only at relatively longer td. In comparison, the kurtosis measurements were relatively insensitive to the injury in the EC in this model, which indicated different sensitivities between diffusivity and kurtosis towards the underlying pathology in this region.Conclusion
The time-dependent change of diffusion kurtosis measured in the mouse brain followed a non-monotonic pattern, and diffusion kurtosis is more sensitive than ADC to ischemic brain injury at short times.Acknowledgements
This work was made possible by the following funding supports: R01HD074593 (JZ) and R21 NS098018 (DW).References
1. Jensen J, Helpern J, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of MRI. Magn Reson Med. 2005;53:1432-1440.
2. Wu E, Cheung M. MR diffusion kurtosis imaging for neural tissue characterization. NMR Biomed. 2010; 23:836-848.
3. Hui S, Fieremans E, Jensen H, et al. Stroke assessment with diffusional kurtosis imaging. Stroke. 2012;43(11):2968-73.
4. Weber A, Hui S, Jensen H, et al. Diffusional kurtosis and diffusion tensor imaging reveal different time-sensitive stroke-induced microstructural changes. Stroke. 2015;46(2):545-50.
5. Does MD, Parsons EC, Gore JC. Oscillating gradient measurements of water diffusion in normal and globally ischemic rat brain. Magn Reson Med. 2003;49:206–215.
6. Wu D, Martin LJ, Northington FJ, Zhang J. Oscillating gradient diffusion MRI reveals unique microstructural information in normal and hypoxia-ischemia injured mouse brains. Magnetic resonance in medicine 2014;72(5):1366-1374.
7. Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. PNAS 2014;111(14): 5088-5093.
8. Novikov DS, Kiselev VG. Effective medium theory of a diffusion-weighted signal. NMR in Biomedicine. 2010; 23(7):682-697.
9. Baron CA, Kate M, Gioia L, Butcher K, Emery D, Budde M, Beaulieu C. Reduction of Diffusion-Weighted Imaging Contrast of Acute Ischemic Stroke at Short Diffusion Times. Stroke. 2015;46(8):2136-2141.
10. Fieremans E, Burcaw LM, Lee H, Lemberskiy G, Veraart J, Novikov DS. In vivo observation and biophysical interpretation of time-dependent diffusion in human white matter. Neuroimage. 2016; 129: 414
11. Pyatigorskaya N, Le Bihan D, Reynaud O, Ciobanu L. Relationship between the Diffusion Time and the Diffusion MRI Signal Observed at 17.2 Tesla in the Healthy Rat Brain Cortex. Magnetic resonance in medicine 2014;72(2):492-500.
12. Fieremans E, Novikov DS, Jensen JH, Helpern JA. Monte Carlo study of a two-compartment exchange model of diffusion. NMR in biomedicine 2010;23(7):711-724.
Figures