0837
A Novel Method for Assessing Myelination with TE dependence of DTI-derived Parameters1Center for Brain Imaging Science and Technology, Zhejiang University, Hangzhou, Zhejiang, China, Hangzhou, People's Republic of China, 2MR Collaboration NE Asia, Siemens Healthcare, Shanghai, China., 3Siemens Healthcare GmbH, Erlangen, Germany., 4Department of Imaging Scienes, University of Rochester, Rochester, NY, USA.
Synopsis
Myelin water is abundant in white matter but myelin signal is often ignored in diffusion models due to its short T2. There is however substantial water exchange between myelin and non-myelin water within typical diffusion times. Using Monte Carlo simulation and in-vivo measurement, we demonstrate that this water exchange might result in an echo-time (TE) dependence of DTI-derived parameters. As myelin water exchange increases with the thickness of myelin sheath, the TE dependence can be used to assess the degree of myelination.
Purpose
Myelin water is abundant in white matter but exhibits very short T2 relaxation (~10 ms) making it invisible for diffusion measuremnts with typical echo times (TE). However, myelin water can still influence the observed signal in a subtle way as AN. Dula et al. have shown that water exchange can mediate the estimated fractions of intra/extra-axonal and myelin water.1 In this study, using a biologically motivated model of myelinated axons in Monte Carlo simulations, we demonstrate that the water exchange between myelin sheath and intra/extra-axonal space might result in TE dependence of DTI-derived parameters. We propose to use this TE dependence to assess the degree of myelination.Methods
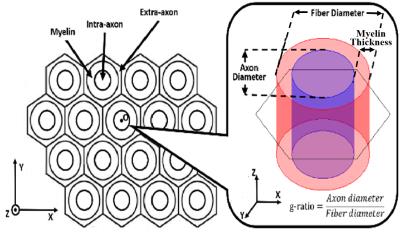
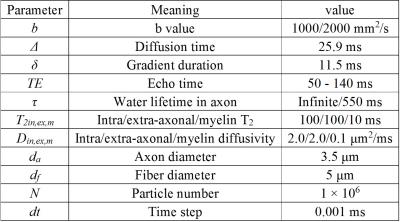
A biophysical model including myelin compartment and allowing inter-compartment exchange was built to simulate the diffusion process in myelinated axons (Fig. 1). The simulation of random walk and synthesis of diffusion signal were performed with Monte Carlo method as described in a previous simulation work.2 The DTI-derived parameters – fractional anisotropy (FA), mean diffusivity (MD), axial diffusivty (DA) and radial diffusivity (DR) - were repeatedly calculated at a set of TEs. The effects of water exchange and different g-ratios (0.6 - 1) were investigated. Other simulation parameters are shown in Table 1.
In order to validate the TE dependence in vivo, two male and three female subjects (aged 23-29 years) were examined on a 3T system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). Each subject was examined with a prototype single-shot PGSE-EPI sequence and the pulse parameters (TE, Δ, δ and b-value) were identical to those used in the simulations. Three white-matter structures (genu, body and splenium of corpus callosum) were outlined for statistical analysis.
Results
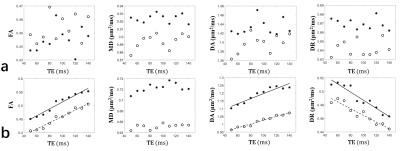
As shown in Fig. 2a, if the lifetime of intra-axonal water was infinite (no water exchange), all DTI-derived parameters had no correlation with TE. As shown in Fig. 2b, if water exchange was allowed, FA and DA had positive correlation with TE while DR had negative correlation with TE.
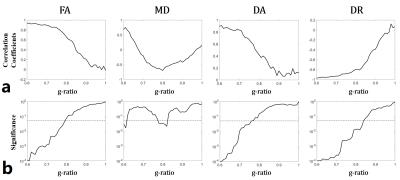
The TE-correlation coefficients and p-value from simulated DTI-derived parameters at different g-ratios are shown in Fig. 3. When the g-ratio was smaller than 0.75 (well myelinated), FA and DA had positive correlation and DR had negative correlation with TE. When the g-ratio reached 0.8, only DR had correlation with TE. When g-ratio was larger than 0.85 (demyelinated), all the DTI-derived parameters had no correlation with TE.
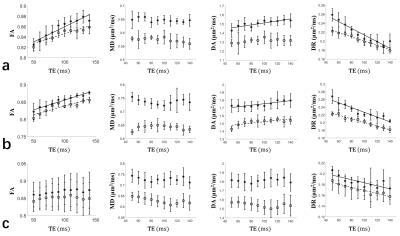
As the in-vivo brain data shows (Fig. 4), in the body of corpus callosum, FA and DA had positive correlation and DR had negative correlation with TE at two b-values (Fig. 4a). In the genu, except DA at b = 2000 mm2/s, the other TE dependences were similar to the body (Fig. 4b). In the splenium, only DR had negative correlation with TE (Fig. 4c).
Discussion
Using Monte Carlo simulations, we demonstrate that, in myelinated axon, the exchange between myelin and intra/extra-axonal water can introduce a dependence of DTI-derived parameters on TE (Fig. 2). This result is consistent with a previous study on rhesus monkeys 3 and our in-vivo human results (Fig. 4). With increasing g-ratio, the correlation of DTI-derived parameters with TE is reduced (Fig. 3). The TE-dependence can be classified into three cases. Case I: FA, DA and DR all have correlation with TE. This indicates well myelinated axon (g-ratio < 0.75). Case II: DR and FA have correlation or only DR has correlation with TE. This indicates moderately myelinated axons. Case III: No parameter has correlation with TE. This indicates demyelinated axons (g-ratio>0.85).
By applying our theory to in-vivo data, we found the genu and body of corpus callosum belong to Case I while the splenium of corpus callosum belongs to Case II, which implies that the splenium is less myelinated than the genu and body. This observation is consistent with a previous study by N. Stikov et al. showing that the g-ratio is higher in the splenium than in the genu and body of macaque corpus callosum.4 Compared with the conventional g-ratio imaging that combines diffusion sensitized imaging (NODDI) and myelin sensitized imaging (magnetization transfer or multicomponent T2 relaxometry), our method reduces the complexity in acquisition and calculation and is thus more practical for clinical applications.
Conclusion
In well myelinated axons, the exchange between myelin and non-myelin water leads to the TE-dependence of DTI-derived parameters. As myelin water exchange is increased with the thickness of myelin sheath, we propose to use the TE-dependence for assessing the degree of myelination, which may potentially serve as a biomarker for demyelination disease like multiple sclerosis and Alzheimer's disease.Acknowledgements
No acknowledgement found.References
1. Dula AN, Gochberg DF, Valentine HL, Valentine WM, Does MD. Multiexponential T2, magnetization transfer, and quantitative histology in white matter tracts of rat spinal cord. Magnetic Resonance in Medicine Official Journal of the Society of Magnetic Resonance in Medicine 2010;63:902-9.
2. Lin M, He H, Schifitto G, Zhong J. Simulation of changes in diffusion related to different pathologies at cellular level after traumatic brain injury. Magnetic Resonance in Medicine 2015;41:130-131.
3. Wen Q, Yu CS, Fan Z, Xiang YD, Jiang H, Yu XY, Li KC. Effects of echo time on diffusion quantification of brain white matter at 1.5T and 3.0T. Magnetic Resonance in Medicine 2009;61:755-760.
4. Stikov N, Campbell JSW, Stroh T, Lavelée M, Frey S, Novek J, Nuara S, Ho MK, Bedell BJ, Dougherty RF. In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage 2015;118:1050-4.
Figures