0835
Hyperpolarized 3He gas MRI in infant lungs: investigating alveolar-airspace size with restricted gas diffusion1Physics, Washington University in St. Louis, St. Louis, MO, United States, 2Center for Pulmonary Imaging Research, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 3Mallinckrodt Institute of Radiology, Washington University in St. Louis, St. Louis, MO, United States, 4Pediatrics, University of Rochester Medical Center, Rochester, NY, United States, 5Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 6Radiology, University of Wisconsin - Madison, Madison, WI, United States, 7Pathology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 8Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
Synopsis
Acinar development in infant humans has not been extensively studied. Hyperpolarized gas diffusion MRI has been shown to relate directly to alveolar-airspace size in adults, pediatrics, canines, and mice. Using ex-vivo lungs from 7 healthy and 1 diseased infant humans, we investigated the relationship between 3He apparent diffusion coefficient (ADC) via mono-exponential decay, alveolar-duct radius via a restricted diffusion model originally developed for mice, and radius via histological measurement. While the mouse model is invalid in the infant diffusion regime, ADC measurements reflect changes in alveolar-airspace size. This method shows promise for longitudinal in-vivo acinar-airway monitoring in neonatal patients.
Purpose
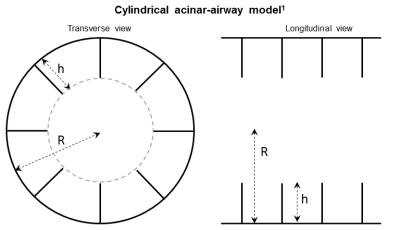
The majority of infants in the neonatal intensive care unit have pulmonary morbidities (e.g. bronchopulmonary dysplasia, congenital diaphragmatic hernia), but the geometry of acinar development in infants has not been extensively studied, largely because of the reliance on post-mortem samples and the lack of non-invasive techniques. Restricted hyperpolarized (HP) gas diffusion MRI is an established research technique that has been shown to relate directly to alveolar-airspace geometry (Figure 1) and size in adults1, pediatrics2, canines3, and mice4, but has yet to be applied to the regime of airway size and geometries expected in infants and preschool pediatric patients. In this study, we investigated the relationship between alveolar-airspace size and restricted 3He gas diffusion via MRI in healthy and diseased ex-vivo infant human lungs.
Methods
Explanted left upper lobes of 7 healthy infants (7.3 ± 6.3 months old, range 0-16 months old, received through the United Network for Organ Sharing) and 1 explanted diseased left lung (filamin A deficiency, 11 months old, from lung transplantation) were imaged on a prototype neonatal-sized multinuclear 1.5T MRI neonatal scanner5. Informed-consent was obtained from subjects’ families. A home-built switched frequency 1H/3He birdcage body coil6 was used to switch between 1H frequency (63.9 MHz) and 3He frequency (48.7 MHz) at 1.5T within ~30 s without disturbing the specimen’s position in the bore. HP 3He gas was prepared from a home-built polarizer and diluted (1:1) with ultra-pure N2 (at this concentration, diffusion coefficient D = 1.2 cm2/s). Absolute nuclear spin polarizations of ~50% were measured by a commercial polarimeter.
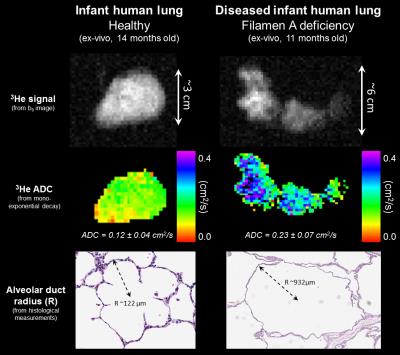
Diffusion-weighted images were acquired (Figure 2 – top row) using a broadband fast gradient-recalled echo pulse sequence with bipolar diffusion-weighted gradient pulses (9 b-values from 0-7.6 s/cm2) during a ~15-sec sustained inflation at 25 cmH2O of pressure. Typical inflation volumes were 10-150 mL. Typical MRI parameters were as follows: flip angle = 6°; TR/TE = ~5.6/3.8 ms; matrix = 64; field of view = ~12 cm; slice thickness = 10 mm; in-plane resolution = ~2.0 mm; and diffusion time = 0.91 ms. Diffusion-weighted images from a specified slice for each specimen were analyzed with a mono-exponential decay model to yield apparent diffusion coefficient (ADC) maps (Figure 2 – second row). Even though an anisotropic, restricted diffusion model has not been developed for the geometry and size regime of neonatal humans, we also attempted to fit the healthy lung data to the existing 3He lung morphometry model developed for healthy adult mice4.
After imaging, lungs were inflation-fixed in formalin at 25 cmH2O. Morphometric measurements of acinar-airway ducts were measured digitally from H&E-stained slides, matched to the specified diffusion slice and corrected for shrinkage during processing. 30 measurements of duct radius (R) (Figure 2 – third row) were averaged for each specimen. 20 measurements of mean linear intercept (Lm) were also averaged for each specimen.
Results
The
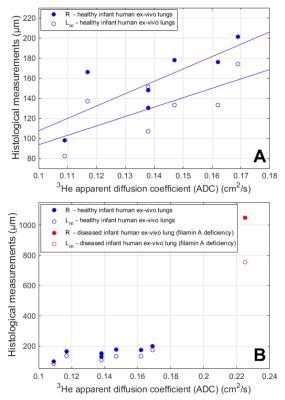
histology-based R values for healthy lungs (mean healthy R = 158 ± 35 µm)
correlated significantly to ADC values (mean healthy ADC = 0.136 ± 0.025 cm2/s)
(Pearson P = 0.037, R = 0.78); correlations between Lm (mean healthy
Lm = 131 ± 29 µm) and ADC were similar (P = 0.042, R = 0.69) (Figure 3). The ADC of the diseased lung
was noticeably larger (0.225 ± 0.074 cm2/s, standard deviation within
specimen), corresponding to an increased alveolar-airway size (R = 1052 ± 225
µm and Lm = 761 ± 273 µm, from histology). The mean percent error
between R values measured in the healthy infant lung explants using the
anisotropic restricted diffusion mouse model and using histological
measurements was 31.8% ± 15.2% (range 3.6%-46.7%).Discussion and Conclusions
These results show that measured ADC values are sensitive to relative changes in acinar-airspace sizes in infant lungs 0-16 months old, by direct comparison to histology. The disagreement between histologically-based and mouse-model-based R measures in healthy infant lungs suggests the 3He lung morphometry model must be extended to the diffusion regime of infant acinar-airways. One possible explanation is that the previously-derived mouse model utilized a diffusion time between 0.3-0.6 ms, while the present experiments were limited to a minimum diffusion time of 0.91 ms.
These ex-vivo results support the potential of using ADC values from HP gas MRI to detect size changes in acinar-airways of neonates in-vivo. Moreover, HP gas MRI has an established safety record in pediatrics2,7 and adults8,9 as a non-invasive and non-ionizing modality that shows translational promise in neonatal patients for monitoring normal and abnormal lung development longitudinally.
Acknowledgements
The authors thank the following sources for research funding and support: The Perinatal Institute at Cincinnati Children’s Hospital Medical Center, The Hartwell Foundation, NIH P01 HL070831, NIH T32 HL007752, NIH T32 CA009206, and NIH U01 HL122700.References
1. Yablonskiy D, Sukstanskii A, Woods J, et al. Quantification of lung microstructure with hyperpolarized 3He diffusion MRI. J Appl Physiol. 2009;107(4):1258-65.
2. Narayanan M, Owers-Bradley J, Beardsmore C, et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med. 2012;185(2):186-91.
3. Hajari A, Yablonskiy D, Quirk J, et al. Imaging alveolar-duct geometry during expiration via 3He lung morphometry. J Appl Physiol. 2011;110(5):1448-54.
4. Wang W, Nguyen N, Yablonskiy D, et al. Imaging lung microstructure in mice with hyperpolarized 3He diffusion MRI. Magn Reson Med. 2011;65(3):620-6.
5. Tkach J, Merhar S, Kline-Fath B, et al. MRI in the neonatal ICU: initial experience using a small-footprint 1.5-T system. AJR Am J Roentgenol. 2014;202(1):W95-W105.
6. Pratt R, Giaquinto R, Ireland C, et al. A Novel Switched Frequency 3He/1H High-Pass Birdcage Coil for Imaging at 1.5 Tesla. Concepts Magn Reson B. 2015;45(4):174-182.
7. Walkup L, Thomen R, Akinyi T, et al. Feasibility, tolerability and safety of pediatric hyperpolarized 129Xe magnetic resonance imaging in healthy volunteers and children with cystic fibrosis. Pediatr Radiol. 2016;46(12):1651-1662.
8. Driehuys B, Martinez-Jimenez S, Cleveland Z, et al. Chronic obstructive pulmonary disease: safety and tolerability of hyperpolarized 129Xe MR imaging in healthy volunteers and patients. Radiol. 2012;262(1):279-89.
9. Lutey B, Lefrack S, Woods J, et al. Hyperpolarized 3He MR imaging: physiologic monitoring observations and safety considerations in 100 consecutive subjects. Radiol. 2008;248(2):655-661.
Figures