0831
Using dynamic MRI to create moving boundary conditions for CFD: determining the causes of upper airway motion in sleep apnea1Division of Pulmonary Medicine, Cincinnati Children's Hospital, Cincinnati, OH, United States, 2Imaging Reseach Center, Cincinnati Children's Hospital, Cincinnati, OH, United States, 3Department of Computing, Imperial College London, London, United Kingdom, 4Division of Radiology, Cincinnati Children's Hospital, Cincinnati, OH, United States
Synopsis
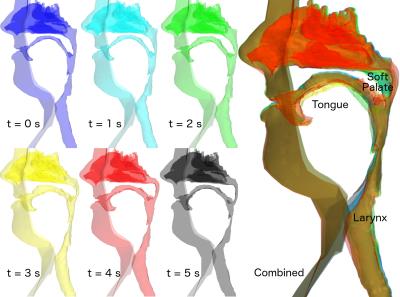
The upper airway consists of complex mobile structures such as the tongue, soft palate and larynx that make predicting surgical outcomes in obstructive sleep apnea difficult. Dynamic computational fluid simulations provide a method to assess the causes of airway deformation, but require information on the airway shape and motion. Combining MR imaging from three sequences provides this information in high spatiotemporal resolution. Simulations allow characterization of airway wall motion as either in the same or opposite direction as the force applied by the intraluminal airflow, which can be used to better understand the subject-specific mechanics of sleep apnea.
Introduction
Obstructive sleep apnea (OSA) in children is due to partial or complete collapse of the upper airway. While a large percentage of children recover from OSA after adenotonsillectomy, many have either persistent or recurrence of OSA post-operatively. To date there is limited understanding of the physiological characteristics of the upper airway that might predict the outcomes of sleep apnea surgeries. An approach that can distinguish between passive motion of the upper airway wall due to intraluminal airflow and active motion due to muscle activity could substantially advance the understanding of the determinants of upper airway collapse.
Computational fluid dynamics (CFD) simulations of airflow that incorporate movement of the upper and descending airway during breathing can provide this information, but require high spatiotemporal resolution of the airway walls. Three MRI sequences were combined to create these boundary conditions, providing high spatial resolution (PD-VISTA), high temporal resolution (dynamic e-THRIVE) and high contrast resolution (UTE). A case study in a healthy volunteer during a breathing maneuver is presented.
Methods
MR imaging was performed on a Philips Ingenia 1.5 T scanner with a 16-channel head and neck vascular coil. 3D Cine-MRI was used to create high temporal resolution images of the airway’s changing shape and position using a T1 high-resolution isotropic volume excitation protocol (e-THRIVE)[α=5°, 96×96×12 matrix, 2.368×2.368×3 mm, TE/TR=0.675/1.905 ms, dynamic time =620 ms for healthy volunteer, =320 ms for clinical cases].
A healthy volunteer (31 Y.O., male) performed a flow-volume-loop breathing maneuver (non-maximal effort) during cine imaging to generate airway motion of similar magnitude to that seen in clinical sleep apnea patients. Flow rates were recorded throughout the dynamic scan using a pneumotachograph. Three sedated free-breathing patients with OSA were then scanned using the same imaging protocol.
High spatial resolution images were obtained using isotropic turbo spin echo imaging (PD-VISTA) to define a surface model of the nasal airways [α=90°, 960×960×200 matrix, 0.35×0.35×0.8 mm, TE/TR=236/1250 ms]. High contrast ultra-short echo time imaging (UTE) was used to segment the descending airway [α=5°, 704×704×68 matrix, 0.5×0.5×2.0 mm, TE/TR=1.51/6.57 ms].
To determine the motion of the airway, each cine-MR was segmented (ITK-snap 3.4.01, Penn Image Computing and Science Laboratory, USA, www.itksnap.org) and a surface mesh constructed. The motion between successive pairs of cine-MR scans was determined by a joined non-rigid registration of each image to the first image (MIRTK 1.1, Department of Computing, Imperial College London, UK, https://biomedia.doc.ic.ac.uk/software/mirtk/). The motion field was then found using conjugate gradient descent to minimize four factors: dissimilarity of each image from the first, difference in surface-node positions, difference in surface-normal vectors, and bending energy of the 4D free-form deformation. The airway position and motion could then be interpolated at any time between the dynamic images.
The UTE and PD-VISTA scans were registered into the position of the first dynamic image and the combined image segmented to create a high-resolution airway surface that was animated using the motion determined from the dynamic scan.
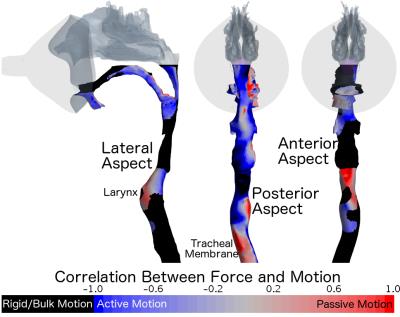
CFD simulations (Star-CCM+ 11.04, Siemens PLM Software, Plano, TX, USA) were performed using the moving high-resolution surface and recorded breathing flow rates as boundary conditions, producing airflow velocity and pressure fields throughout the breathing maneuver. Comparing airway wall motion with the pressure and shear forces from the airflow allows the motion to be classified as either active or passive.
Results
During the breathing maneuver, the tongue and soft palate move most, causing a reduction in local luminal cross-sectional area during expiration: 71% and 84% respectively (Figure 1).
Figure 2 shows the correlation between force and motion in the airway. The anterior larynx and posterior tracheal membrane move as expected; in the direction of the force applied by the airflow (passive motion), whilst the airway through the mouth, hyoid cartilage and anterior trachea is dominated by active bulk motion of the head or chest. The motion of the tongue and soft palate are also opposite to the forces created by airflow (active motion). The oral airway actively expands during inhalation to reduce resistance, rather than collapsing under the applied low pressure.
Conclusions
CFD simulations have been performed with dynamic boundary conditions derived from multiple MRI sequences. Regions of airflow with high resistance in the dynamic airways and muscle driven motion were identified. In patients, this information will allow surgeons to better understand the motion and interior airflow of the airways, which may allow more tailored surgeries to the specific problems of each airway. Previous simulation methods could not have calculated this behavior, as static CFD ignores airway wall motion, whilst fluid-structure-interaction only allows passive motion.Acknowledgements
No acknowledgement found.References
1. Paul A. Yushkevich, Joseph Piven, Heather Cody Hazlett, Rachel Gimpel Smith, Sean Ho, James C. Gee, and Guido Gerig. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006 Jul 1;31(3):1116-28.Figures