0829
Assessment of cystic fibrosis disease using UTE imaging with XD-GRASP reconstruction: a comparison with CT1Department of Radiology, University Hospital (CHUV) and University of Lausanne (Unil), Lausanne, Switzerland, 2Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, New York University School of Medicine, New York, NY, United States, 3Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 4Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 5Adult CF multisites unit, Hospital of Morges, Morges, Switzerland, 6Service of Pneumology, Department of Medicine, University Hospital (CHUV), Lausanne, Switzerland
Synopsis
Motion resolved reconstructions, using compressed sensing, of 3D ultra short echo time (UTE) acquisitions in cystic fibrosis patients were performed. The definition of the lung-liver interface was quantified and found to be significantly higher than that in motion corrupted reconstructions of the whole datasets. The Helbich-Bhalla score for cystic fibrosis was determined using both computed tomography (CT) and MRI data. Correlation between scores obtained with both modalities was good (ρ=0.77) but consistency was moderate (ICC=0.62). This was due to average MRI scores being lower by 15%, most likely because mosaic perfusion could not be assessed with MRI.
Purpose
Cystic fibrosis patients require frequent imaging of their lungs. Magnetic resonance imaging (MRI) offers a promising radiation-free alternative to computed tomography (CT). Ultra-short echo time (UTE) imaging can overcome signal loss due to the short T2* in lung tissue1. In this work, to avoid motion corruption of the images, we studied the performance of a respiratory motion-resolved technique that retrospectively sorts UTE data into respiratory phases and exploits compressed sensing reconstruction for respiratory motion suppressed free-breathing 3D imaging of the lungs in cystic fibrosis patients. Also, diagnostic performance was compared to that of CT.Methods
A prototype 3D radial double echo (TE1=0.05, TE2=2.86) UTE sequence2 with spiral phyllotaxis3 trajectory was used to acquire data (67530-90300 k-space lines) in 7 adult cystic fibrosis patients (4 male, mean age: 39 years) at 1.5T (MAGNETOM Aera, Siemens Healthcare). The superior-inferior component of respiratory motion was detected in the second echo superior-inferior projections2. This signal was then used to reconstruct image volumes (1.3mm isotropic resolution, 500mm field of view) with 2 separate methods: In the first case, expiratory and inspiratory images (50% of total data used for each dataset) were reconstructed using gridding (standard non-uniform Fourier transform). In the second approach, data were binned in 2-4 respiratory bins and images reconstructed using a compressed sensing algorithm, exploiting sparsity along the respiratory dimension (eXtra-Dimensional Golden-angle Radial Sparse Parallel: XD-GRASP)4-6. Additionally, motion corrupted images were reconstructed by gridding of the full data sets acquired.
The sharpness of the transition at the lung-liver interface was assessed by computing the normalized maximum of the first derivative7 (MD) on the end-expiratory images. The maximum of the first derivative was computed along 21 vertical profiles going through the lung-liver interface. The value measured in each profile was then normalized to the value measured in the corresponding profiles of the motion corrupted datasets, so that a value larger than 1.0 indicates an improvement in image definition. Statistical significance was evaluated using a one-sample t-test. The MD values obtained with gridding and XD-GRASP were compared and statistical significance was assessed with a paired two-sample t-test.
UTE images were reviewed by a chest imaging expert (C.B., 25 years experience) and the Helbich-Bhalla8 score was computed using XD-GRASP end-expiration images. The same process was applied to images obtained with the most recent clinically indicated CT. Intra-class correlation for consistency (ICC), Spearman’s ρ and mean percentage error were computed and Bland-Altman analysis was performed.
Results
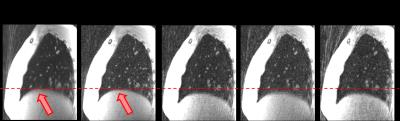
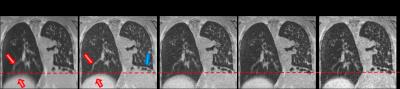
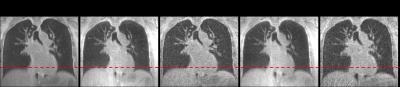
Data acquisition and image reconstruction were performed successfully for all patients. Respiratory motion could be resolved (figure 1 and 2). Visually, the diaphragm appeared better defined, and blurring of lung vessels was reduced compared to the full dataset. Diaphragm positions were more clearly separated in XD-GRASP reconstructions compared to gridding reconstruction (figure 3).
MD was higher than 1.0 in 103/147 profiles when gridding was used and in 116/147 profiles when using XD-GRASP. The average MD over all patients was 1.1 (P=0.004) when using gridding reconstruction and 1.3 (P=0.004) when XD-GRASP was used. The difference in MD measured with gridding compared to XD-GRASP was not significant (P=0.1).
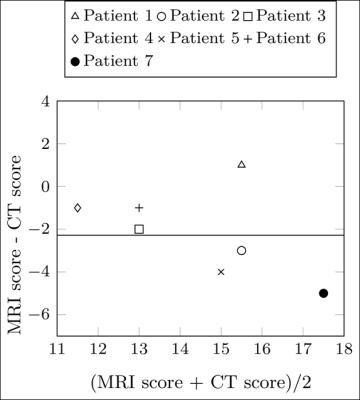
Correlation between Helbich-Bhalla scores measured using XD-GRASP MRI and CT was good (ρ=0.77) but ICC was moderate (ICC=0.62). The mean score was 15% lower (bias of 2.3, figure 4) when using MRI (13.3), compared to CT (15.6).
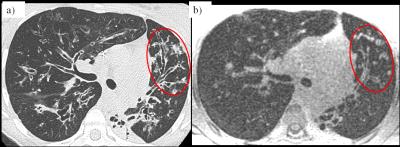
Bronchiectasis, mucous plugging (figure 5) and alveolar consolidations (figure 2) could be well visualized. Distally, assessment of airways was more difficult due to the larger voxel size of UTE images compared to CT. Severity of mosaic perfusion could not be assessed on the UTE images due to background noise in the parenchyma.
Discussion and Conclusion
The method studied in this work, by separating the different phases of respiratory motion, allowed significant improvements in image sharpness to be measured at the lung-liver interface. The use of XD-GRASP led to higher observed MD values than regular gridding but statistical significance was not reached.
The comparison with CT showed good correlation between both modalities but with a tendency for MRI to underestimate the Helbich-Bhalla score, as the mean bias between the two methods was 2.3. This bias can most likely be attributed to the inability to assess mosaic perfusion on MRI datasets. Further studies are required to assess the respective influence of acquisition and reconstruction methods on the ability to assess subtle signal differences in parenchymal signal.
These results confirm the possibility to obtain clinically useful information while minimizing radiation exposure in cystic fibrosis patients. An added advantage of this method is the absence of breath holds, which may increase patient comfort and compliance.
Acknowledgements
This work was supported by the Swiss National Science Foundation grant 320030_143923 as well as a grant from the Fondation BCV.References
1. Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: projection reconstruction MR imaging. Radiology 1991;179:777–781.
2. Delacoste J, Chaptinel J, Beigelman C, Piccini D, Sauty A, Stuber M. A Double Echo Ultra Short Echo Time Acquisition for Respiratory Motion Suppressed High Resolution Imaging of the Lung. In: Proceedings of the 23rd Annual Meeting of ISMRM, Toronto, Ontario, Canada, 2015.
3. Piccini D, Littmann A, Nielles-Vallespin S, Zenge MO. Spiral phyllotaxis: The natural way to construct a 3D radial trajectory in MRI. Magn Reson Med 2011;66:1049–1056. doi: 10.1002/mrm.22898.
4. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med 2016;75:775–788. doi: 10.1002/mrm.25665.
5. Piccini D, Feng L, Bonanno G, Coppo S, Yerly J, Lim RP, Schwitter J, Sodickson DK, Otazo R, Stuber M. Four-dimensional respiratory motion-resolved whole heart coronary MR angiography. Magn Reson Med 2016. doi: 10.1002/mrm.26221.
6. Feng L, Delacoste J, Chandarana H, Piccini D, Girvin F, Stuber M, Sodickson DK, Otazo R. Four-Dimensional Respiratory Motion-Resolved Sparse Lung MRI. In: Proceedings of the 24th Meeting of ISMRM, Singapore, 2016.
7. Tibiletti M, Paul J, Bianchi A, Wundrak S, Rottbauer W, Stiller D, Rasche V. Multistage three-dimensional UTE lung imaging by image-based self-gating. Magn Reson Med 2016;75:1324–1332. doi: 10.1002/mrm.25673.
8. Helbich TH, Heinz-Peer G, Eichler I, Wunderbaldinger P, Götz M, Wojnarowski C, Brasch RC, Herold CJ. Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology 1999;213:537–544. doi: 10.1148/radiology.213.2.r99nv04537.
Figures