0817
The comparative study of MR renography versus 99mTc-DTPA renal scintigraphy for determining split renal function of the transplanted kidney1Center for Medical Device Evaluation, CFDA, Beijing, People's Republic of China, 2MR Research China, GE Healthcare, Beijing, People's Republic of China, 3Department of Radiology, the First Affiliated Hospital with Nanjing Medical University, Nanjing, People's Republic of China
Synopsis
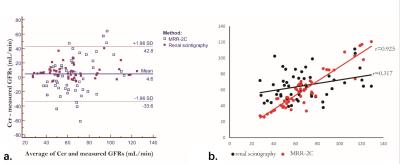
The evaluation of split kidney function is of clinical significance for patients after renal transplantation. This study explored two available approaches, dynamic MR renography vs. 99mTc-DTPA clearance, for determining split renal GFR in 57 transplanted patients. MR-based GFR was measured using an established Baumann-Rudin model, modified two-compartment and three-compartment model. MR results were compared with 99mTc-DTPA, using 24-h creatinine clearance rate as a reference. MRR using a 2C model had significantly higher accuracy (r = 0.925) than 99mTc-DTPA clearance (r = 0.317), thus could be a more promising way for evaluation of transplanted kidney function.
INTRODUCTION
The application of 99mTc-DTPA renal scintigraphy has validity for renal glomerular filtration rate (GFR) assessment in home position. Unfortunately, the kidney depth may be varied among individuals after renal transplantation, which hampering the normalization of background tissue signal and the clinical reproducibility 1. Alternatively, low-dose 3D MR renography (MRR) with high spatial resolution has been proposed to evaluate the renal function 2. However, to date, the diagnostic value of 3D MRR and 99mTc-DTPA renal scintigraphy has never been compared for the determination of split kidney function in transplanted patient. This study aims to compare the performance of GFR evaluation after renal transplantation between the two methods, using creatinine clearance rate (Ccr-GFR) as a reference standard.METHODS
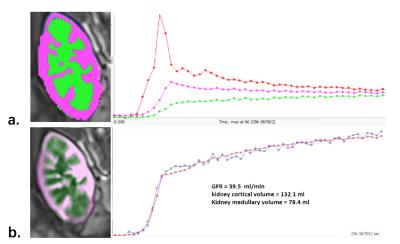
This IRB-approved prospective study included 57 patients who underwent renal transplantation. MR renography was performed at 3.0 T with three-dimensional gradient-echo imaging (4.0 s/phase, 4.5 min; 4 ml Gd-DTPA with injection rate of 3.5ml/s) one week after the surgery. Nuclear renal scintigraphy was performed 3 days after the MRR using a standard 99mTc-DTPA clearance. The MRR data were plotted with an established Baumann-Rudin model (BR) 2, modified two-compartment (2C) 3, and three-compartment (3C) model 4, respectively, for GFR estimation based on a dedicated in-house software (FireVoxel; CAI2R, New York University) (Figure 1). Ccr-GFR was measured by collecting 24-h urine creatinine (u-Cr), urinary production (U) and serum creatinine (s-Cr) level ( Ccr = u-Cr × U / s-Cr ). Pearson correlation coefficients and Bland-Altman plots were adopted to test the inter-class concordance between the three methods.RESULTS
The results of human study demonstrated that mean standard Ccr-GFR was 67.1 ml/min (range, 28.6 - 121.9 ml/min). GFR obtained by MRR-2C (58.9 ml/min; range, 27.5 - 120.7 ml/min) shows the highest correlation coefficients (r = 0.925) with the standard Crr-GFR, followed by MRR-BR (44.9 ml/min; range, 16.3 - 89.9 ml/min; r = 0.471), 99mTc-DTPA renal scintigraphy (65.2 ml/min; range, 37.8 - 111.5 ml/min; r = 0.317), and MRR-3C (32.3 ml/min; range, 16.7 - 40 ml/min; r = 0.134), respectively. MRR-2C model produced significantly smaller bias in determination of transplanted kidney GFR than 99mTc-DTPA renal scintigraphy (MRR-2C: 7.4 ± 9.5 ml/min vs 99mTc-DTPA: 0.65 ± 25.1 ml/min, p < 0.001) (Figure 2).CONCLUSION
In this study, a tracer kinetic-based MRR approach was developed to measure renal GFR in transplanted patients. Based on our comparative analysis, the MRR using a modified 2C model shows predominantly higher accuracy than other two MRR models and 99mTc-DTPA renal scintigraphy in determination of transplanted renal GFR. The advantage of MRR is that the high spatial resolution of MR imaging provides the ability for directly determination of renal hemodynamic characteristics and segmentation of renal cortex and medulla. Another potential reason of the enhanced reproducibility is the simplified consideration of the artery input function.Acknowledgements
No acknowledgement found.References
1. Heaf JG, et al. Uses and limitations of renal scintigraphy in renal transplantation monitoring. European journal of nuclear medicine. European journal of nuclear medicine. 2000.
2. Louisa Bokacheva, et al. Estimates of glomerular filtration rate from MR renography and tracer kinetic models. JMRI 2009.
3. J. L. Zhang, e t al. Assessment of renal function using MR Renography without aortic input information. ISMRM 2008.
4. Yamamoto A, et al. Quantitative evaluation of acute renal transplant dysfunction with low-dose three-dimensional MR Renography. Radiology 2011.
Figures