0816
An MR compatible kidney perfusion system to assess kidney function and organ preservation.1Medical Imaging, University of Arizona, Tucson, AZ, United States, 2Electrical and Computer Engineering, University of Arizona, Tucson, AZ, United States, 3Physiological Sciences, University of Arizona, Tucson, AZ, United States, 4Siemens Medical Solutions USA, Inc., United States, 5Surgery, University of Arizona, Tucson, AZ, United States, 6Radiology and Imaging Sciences, Emory University Hospital, Atlanta, GA, United States
Synopsis
A MR-compatible kidney perfusion system is presented to assess the quality of kidney preservation schemes for transplantation. DCE-MRI is used to estimate and compare the glomerular filtration rate (GRF) in kidneys following a 24 hrs cold gaseous oxygen perfusion (persufflation) Vs a standard 24 hrs cold ischemic storage.
Target:
Investigators studying renal perfusion and organ preservation techniques for kidney transplant.Background:
In 2014, 67% of the 17105 kidney transplants performed in the US came from deceased donors [1]. Moreover, due to a limited organ supply, the number of transplants using organs exposed to prolonged ischemia time has been increasing. It is crucial to develop methods to assess the quality of various kidney preservation schemes such as cold perfusion, persufflation or cold storage. We present a NMR-compatible porcine kidney perfusion system designed to be used on a clinical scanner using a standard MR coil and an easy to set-up perfusion circuit (Fig 1). In this report we used dynamic contrast-enhanced MRI to estimate and compare the glomerular filtration rate (GRF) in porcine kidneys undergoing two kidney preservation protocols: 1) a standard 24 hrs cold (4 oC) ischemia storage and 2) a 24 hrs cold gaseous oxygen perfusion called persufflation [2].Methods:
Perfusion system: The perfusion system consists of a bath circulator (Thermo Haake ARCTIC AC200 A25), two Masterflex peristaltic pumps (Cole-Parmer), an autoclavable oxygenator device, and a Lifeport device (Organ Recovery Systems). All kidneys were obtained from heparinized porcine donors after 30 minutes of warm ischemia to mimic typical conditions of procurement from deceased donors. Following procurement and cannulation of the kidney artery, vein and ureter, the porcine kidneys were either stored at 4 oC (ischemic cold storage) or connected to a persufflation device [2] operating at 4 oC for 24 hrs prior imaging. For imaging, persufflated and ischemic kidneys were secured in the LifePort cradle and cold-perfused (7 oC) with a preservation solution (SPS-1) from Cell & Tissue Systems. The cradle was inserted in a commercial Head and Neck RF coil as shown in Fig 1.
MRI protocol: All imaging was performed on a 3T Skyra scanner (Siemens). Dynamic contrast-enhanced MRI (DCE-MRI) was performed using a golden angle stack-of-star radial acquisition (radial-Vibe, Siemens). Total acquisition time was 4 min. A Gadolinium-based contrast agent (Multihance, 10 ml@4mM) was injected after 20 sec of acquisition in the cannulated artery. Temporal windowing was used to group k-space data to a fixed temporal resolution while golden angle radial acquisition ensured a good coverage of k-space. Images were reconstructed using Compressed Sensing reconstruction with total variation applied in temporal dimension [3] as shown in following equation.$${d={(}{\arg\min_{d}\ }\|{F.C.d-m}\|}^2_{2}+\lambda\|S.d\|_{1}{)}$$
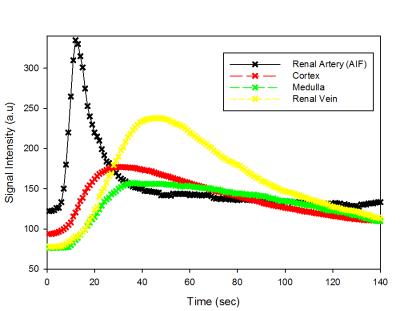
A Modified Look-Locker Inversion recovery method (MOLLI) was used to generate T1 map pre and post injection of a 4mM Gad-DTPA solution. Manual segmentation was done on CS reconstructed images using only 21 lines of k-space (2 sec temporal resolution). The arterial input function (AIF) curve was generated using the signal from an ROI placed on renal artery and the kidney signal was obtained by segmentation of the kidney parenchyma (cortex + medulla). The AIF and parenchyma signal were used as input to the two-compartment pharmacokinetic model to extract an averaged GFR per unit volume of kidney (Ktrans) [4]. Data were continuously collected for total of 4 min but only fitted over a 90 sec acquisition window from the arrival of contrast.
Results:
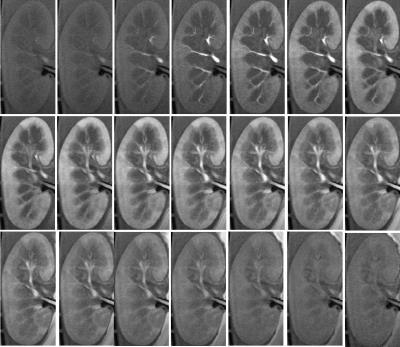
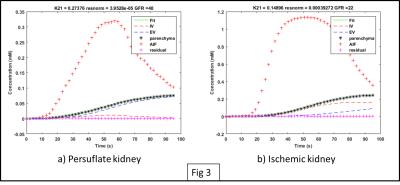
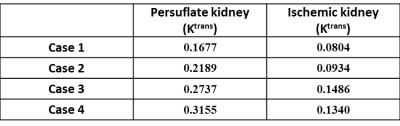
Fig. 2 shows a series of reconstructed images from a persufflated “ex vivo” kidney at different times point following the contrast agent injection. Fig. 3 provides the signal intensity-time delay curve for ROI’s drawn on artery (black), cortex (red) , medulla (green) and vein (yellow) showing difference between the contrast arrival. Fig. 4 shows the non-linear least square curve fits using modified the two-compartment model and the corresponding Ktrans values for persufflated (a) and ischemic kidney (b). Table 1 shows Ktrans values of persufflated vs ischemic porcine kidney for four ex vivo cases. Paired t-test done on the dataset shows statistically significant higher Ktrans values for persufflated kidneys compared to ischemic kidney (p< 0.05).Conclusion:
Kidney persufflation was shown to be associated with an increase of GFR values as measured by DCE-MRI compared to GFR values measured in standard cold ischemic conditions. Higher GFR value suggest better preservation conditions for persufflated kidneys. Current studies aim to investigate kidney filtration rate, viability, and function prior to transplantation comparing various schemes of kidney preservation techniques.Acknowledgements
NIH SBIR P2 R44 DK070400.References
1. http://optn.transplant.hrsa.gov/
2. Thomas M. Suszynski, Michael D. Rizzari, William E. Scott III, Linda A. Tempelman, Michael J. Taylor, Klearchos K. Papas, Persufflation (or gaseous oxygen perfusion) as a method of organ preservation, Cryobiology, Volume 64, Issue 3, June 2012, Pages 125-143.
3. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R: Golden-Angle Radial Sparse Parallel MRI: Combination of Compressed Sensing, Parallel Imaging, and Golden-Angle Radial Sampling for Fast and Flexible Dynamic Volumetric MRI. Magnetic resonance in medicine , 2014, 72(3):707-717.
4. Tofts PS, Cutajar M, et al: Precise measurement of renal filtration and vascular parameters using a two-compartment model for dynamic contrast-enhanced MRI of the kidney gives realistic normal values. European Radiology 2012, 22(6):1320-1330.
Figures