0813
Pubertal contributions to white matter apparent fibre density in late childhood: a fixel-based analysis1Department of Paediatrics, The University of Melbourne, Melbourne, Australia, 2Developmental Imaging, Murdoch Childrens Research Institute, Melbourne, Australia, 3The Florey Institute of Neuroscience and Mental Health, Melbourne, Australia, 4Department of Medical Education, The University of Melbourne, Melbourne, Australia, 5Discipline of Psychiatry, The University of Sydney, Sydney, Australia
Synopsis
Recent evidence supports the contribution of pubertal stage to local and global grey and white matter remodelling. Using fixel-based analyses, we show that pubertal children have greater apparent fibre density in the splenium of the corpus callosum compared with age-matched pre-pubertal children. This finding suggests that pubertal onset itself, rather than chronological age, drives the remodelling of white matter microstructure – which is an important consideration for assessing biological age. This is particularly important for studying paediatric and adolescent populations, as pubertal stage may be an important factor to consider in addition to chronological age.
Purpose
A growing number of studies have identified that relative early/late onset of puberty can lead to differential patterns of microstructural white matter development[1, 2], but disentangling age-related brain development from pubertal processes has proved challenging. To date, studies of white matter organisation over the pubertal period have been limited to diffusion tensor modelling, which is inherently non-specific to the mechanisms of microstructural change. The aim of this study was to investigate white matter properties between age-matched pre-pubertal and pubertal children using fixel-based analysis (FBA)[3], a recently developed method that estimates the microscopic density and macroscopic cross-section of a fibre bundle. We hypothesised that children with physical signs of puberty would have widespread higher white matter apparent fibre-density (FD), fibre cross-section (FC), and fibre density and cross-section (FDC).Methods
Diffusion-weighted Magnetic Resonance Imaging (MRI) data was acquired for 74 typically developing children (M=10.4, SD=0.43 years, 31 female) on a 3.0T Siemens Tim Trio system (60 diffusion directions, b-value=2800 s/mm2, voxel-size=2.4 mm3, TE/TR: 110/3200 msec, 4 b0’s). Diffusion data were processed using the MRtrix3 software package (http://www.mrtrix.org) using the standard FBA processing pipeline.
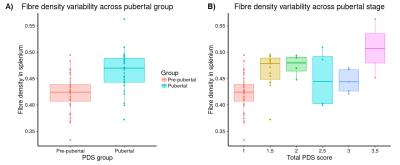
Pubertal stage was assessed via The Pubertal Development Scale (PDS) which assesses maturation of secondary sexual characteristics such as body hair and skin changes, and a total puberty score ranging from 1-5 was generated using the Shirtcliff method[4]. Children were subsequently split into pre-pubertal (absence of physical signs of puberty, where PDS=1)(N=44, M=10.4 years) and pubertal (at least one physical sign of puberty, where PDS>1)(N=30, M=10.5 years) groups for FBA.
General Linear Models were computed for permutation-based testing of FBA metrics using connectivity-based fixel enhancement[5], and any significant region of interest (ROI) was extracted at pFWE < .05 for post-hoc statistical analyses.
Results
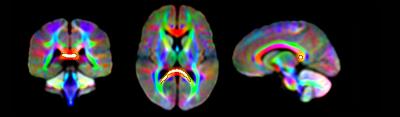
Significant fixel-wise differences in FD were observed between the pubertal groups, covarying for age and sex. The pubertal group had significantly higher FD (pFWE < .001) compared with age-matched pre-pubertal children (Fig. 1-3), localised to the splenium of the corpus callosum. There were no significant fixel-wise group differences in FC and FDC between the groups (pFWE > .05). Post-hoc analyses on mean FD in the significant ROI revealed group differences between the pubertal groups: [95% CI of difference] = [-.06, -.02] (Fig. 4), and this finding was replicated using mean FD in the entire white matter mask. Categorical regression between FD and PDS score was also significant (Adjusted R2 = .28, p < .001). Interaction effects of age and sex on pubertal group were non-significant for all fibre metrics, so homogeneity of regression was not violated for the FBA. In addition, only a significant main effect of sex on FC was found, however given group differences were only present in FD this result was not further explored.Discussion
The finding of higher FD in the pubertal group may signify that the onset of puberty results in increased white matter FD in the splenium, independent of chronological age. Maturation of corpus callosum connections leads to more efficient interhemispheric communication, which is an important step in pubertal onset as a number of processes accompany pubertal progression such as the development of executive function and emotion regulation. White matter follows a posterior-to-anterior gradient of development[6], and our findings attribute these earlier posterior developmental alterations to pubertal onset. Previous work has linked further pubertal advancement with the corpus callosum midbody shape[7], which is in line with this gradient of development.
The absence of group differences in FC could be attributed to unchanged fibre bundle cross-section across pubertal onset. Higher FD with unchanged fibre cross-section can be interpreted as increased axonal count in fibre bundles, therefore pubertal onset may result in axons being laid down in a fibre bundle of unchanged cross-sectional area. The absence of interaction effects of age and sex on pubertal group adds to the evidence that pubertal onset itself may be driving these white matter differences, independent of age-related and sex-dependent mechanisms of typical brain development. Future studies should investigate the longitudinal course of white matter development over adolescence, and relate this to pubertal development indexed by physical maturation as well as more specific markers of puberty such as circulating endogenous adrenal and gonadal hormone levels.
Conclusion
We show evidence that pubertal onset, evidenced by maturation of secondary sexual characteristics, initiates the remodelling of white matter. Pubertal onset leads to increases in apparent fibre density in the splenium independent of sex and age, and may be an important metric to investigate over pubertal development, particularly for group studies where age-matched clinical and typical populations may be at various stages of puberty.Acknowledgements
This research was conducted within the Developmental Imaging research group, Murdoch Childrens Research Institute and the Children’s MRI Centre, The Royal Children's Hospital, Melbourne, Victoria. It was supported by the Murdoch Childrens Research Institute, The Royal Children’s Hospital, Department of Paediatrics at The University of Melbourne, and the Victorian Government's Operational Infrastructure Support Program. The project was generously supported by RCH1000, a unique arm of The Royal Children’s Hospital Foundation devoted to raising funds for research at The Royal Children’s Hospital.References
1. Herting, M.M., et al., The Impact of Sex, Puberty, and Hormones on White Matter Microstructure in Adolescents. Cerebral Cortex, 2012. 22(9): p. 1979-1992.
2. Asato, M.R., et al., White Matter Development in Adolescence: A DTI Study. Cerebral Cortex, 2010. 20(9): p. 2122-2131.
3. Raffelt, D.A., et al., Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage, 2016.
4. Shirtcliff, E.A., R.E. Dahl, and S.D. Pollak, Pubertal development: correspondence between hormonal and physical development. Child development, 2009. 80(2): p. 327-337.
5. Raffelt, D.A., et al., Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage, 2015. 117: p. 40-55.
6. Krogsrud, S.K., et al., Changes in white matter microstructure in the developing brain-A longitudinal diffusion tensor imaging study of children from 4 to 11 years of age. Neuroimage, 2016. 124: p. 473-486.
7. Chavarria, M.C., et al., Puberty in the corpus callosum. Neuroscience, 2014. 265: p. 1-8.
8. Dhollander, T., et al., Time to move on: an FOD-based DEC map to replace DTI's trademark DEC FA. Proc ISMRM, 2015. 23: p. 1027.
Figures
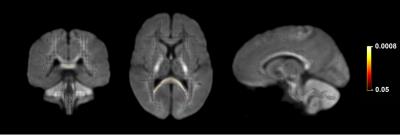
Fixels with significantly higher FD in the pubertal group compared with the pre-pubertal group. Fixel analysis mask (black), with colour-coded significant fixels (pFWE < .05) overlaid on the population-based fibre orientation distribution (FOD) template.