0811
Novel Functional Brain Development Patterns during Infancy Revealed through the Application of Functionally Derived Brain Parcellations1Biomedical Imaging Research Institute, Cedars Sinai Medical Center, Los Angeles, CA, United States, 2Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
Signal heterogeneity within the predefined regions of interest (ROIs) may confound the functional connectivity estimation. In this study, we generate brain parcellations for neonate, 1-year, and 2-year-old infants, respectively, and use them to reveal potential novel functional developmental patterns. Our results show the progression of local functional specialization during early brain development. Moreover, different patterns of hub distributions are observed using different functional parcellation schemes suggesting the importance of selecting appropriate functionally-derived brain parcellations in characterizing infant whole brain connectivity pattern.
Purpose
It is now well known that functional connectivity estimation can be seriously contaminated by signal heterogeneity within the predefined regions of interest (ROIs). Therefore, a number of functionally derived atlases have been proposed in adult studies to improve signal homogeneity within ROIs and facilitate more accurate functional connectivity pattern characterization. In early brain development studies, functionally derived brain parcellations were lacking until our recent attempt to build such a set of parcellations in infants aged between neonates and two years. In this study, we aimed to demonstrate the potential novel functional developmental patterns through the application of this set of functional brain parcellations in infants.Methods
Our functional brain parcellations were derived from rsfMRI data from 238 neonates (136 females), 148 1-year-old (80 females), and 111 2-year-old (51 females) 1,2. Briefly, after preprocessing 3 and scrubbing-based motion artifact correction 4, a hybrid iterative normalized cut algorithm (HI-NCUT) and a novel similarity-based stopping criterion to determine the number of functional parcellations for each AAL region 5 in each age group (instead of pre-defining them based on prior knowledge), offering the opportunity to objectively examine the progress of local functional specialization (i.e., revealed by the increasing number of functional sub-units with distinguishable whole brain connectivity patterns within a certain AAL area).
We first examined the characteristics of functional sub-units within each AAL area (e.g., number, location, and associated global functional connectivity patterns) and their changes across the first two years of life. Second, a functional specialization index was derived to quantify the changes in local functional specialization. Specifically, the 2-year final sub-unit division map within each AAL area was used as a template and warped back to neonates and 1-year olds to calculate the mean cross-correlations of their whole-brain functional connectivity patterns. Subsequently, 1 minus the mean correlation was calculated as functional specialization Index (FSI) which ranged from 0 to 1 with higher value indicating higher levels of functional specializations across different functional sub-units. Finally, the developmental changes in whole brain functional topography was characterized in terms of hub distributions based on betweeness-centrality measures through graph-theoretical analyses. The derived distributions were also compared across different brain parcellation schemes (i.e., CC et al. 6, and YJ et al. 7).
Results
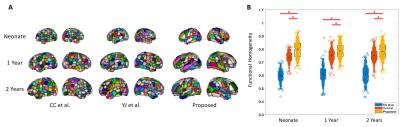
Fig. 1A shows our functional brain parcellations for neonate, 1 year, and 2 years groups, with 227, 253, and 278 functionally derived subunits, respectively. Fig. 1B demonstrated the significantly higher levels of within-unit functional homogeneity obtained from our parcellation schemes compared with other schemes.
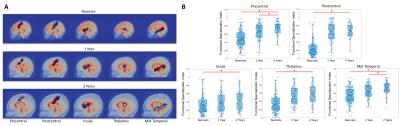
Fig. 2A shows the subunit topologies in precentral, postcentral, insula, thalamus, and mid-temporal areas in the three infant groups as examples of the evolution of local functional topologies during infancy. Specifically, all areas except the insula demonstrated increased number of subunits corresponding to increasingly finer separations between local functional areas that are known to be specialized in adults. For example, the pre/postcentral gyrus became more differentiated along the medial-lateral axis that is highly consistent with the sensorimotor homunculus layout. Importantly, when examining the whole brain functional connectivity maps associated with each sub-unit, increasingly more differentiable patterns were also observed with age (except for the insula for which highly consistent anterior-posterior separations were observed from all three groups). Indeed, our quantitative FSI measures (Fig. 2B) showed significant increases with age except for the insula area. Overall, 51 out of 90 AAL regions demonstrated increasing number of sub-units together with significantly increased FSI index during infancy, indicating a general trend of functional specialization between adjacent brain areas during infancy.
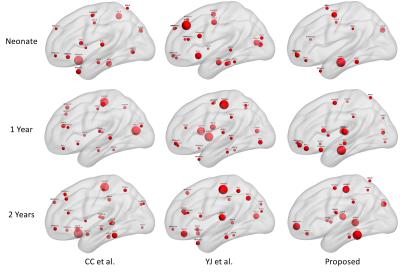
Finally, Fig. 3 illustrates the hub distributions derived from our functional parcellations and two other parcellation schemes. Noticeable differences were observed across different parcellation schemes indicating the sensitivity of such characterization against ROI selections. Specifically, compared with other schemes, the hub distribution derived from our functional parcellations demonstrated better continuity across ages featured by dominant primary sensorimotor, visual, and auditory hubs with progressively more emphasis on frontal structures.
Discussion
Through the derivation and characterization of functional brain parcellations during infancy, our results confirmed the progression of local functional specialization during early brain development. More importantly, our findings of dramatically different hub distributions based on different functional parcellation schemes emphasize the importance of selecting appropriate functionally-derived brain parcellations as ROIs in characterizing infant whole brain connectivity pattern and graph-theoretical measures.Acknowledgements
This study was supported by NIH grants (R01MH064065, R01HD05300 to JHG; R21NS088975, R03DA036645 to WG) and Cedars-Sinai Institutional Support to WG.References
1 Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, Zhu H, Hamer RM, Styner M, Shen D. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cerebral Cortex 2012; 22:2478-2485.
2 Gao W, Lin W, Grewen K, Gilmore JH. Functional Connectivity of the Infant Human Brain Plastic and Modifiable. The Neuroscientist 2016; 1073858416635986.
3 Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage 2012; 62:782-790.
4 Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron 2011; 72:665-678.
5 Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, Shen D. Infant brain atlases from neonates to 1- and 2-year-olds. PLOS One 2011; 6:e18746.
6 Craddock RC, James GA, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp 2012; 33:1914-1928.
7 Jin Y, Wee CY, Shi F, Thung KH, Ni D, Yap PT, Shen D. Identification of infants at high-risk for autism spectrum disorder using multiparameter multiscale white matter connectivity networks. Hum Brain Mapp 2015; 36:4880-4896.
Figures