0807
Anatomically constrained tractography and structural connectome of the neonatal brain1MRC Centre for Reproductive Health, University of Edinburgh, edinburgh, United Kingdom, 2Centre for Clinical Brain Sciences, University of Edinburgh, edinburgh, United Kingdom, 3Clinical Research Imaging Centre, University of Edinburgh, edinburgh, United Kingdom
Synopsis
The Anatomically-Constrained Tractography framework (ACT) is an advanced method to create tractography that relies on very accurate segmentation of the brain. To evaluate its role in tractography of the developing brain, we optimised the pipeline for neonatal data and compared against other methods (FA and WM seeding, with deterministic and probabilistic algorithms) used for the computation of neonatal structural connectivity. The results suggest that ACT with seeding at the GM / WM interface enhances anatomic accuracy of neonatal tractography, compared with other methods, and it has a significant impact on network measures.
PURPOSE:
The Anatomically-Constrained Tractography framework (ACT)1 is one of the most advanced methods to create tractography data, relying on very accurate segmentation and parcellation of the brain. In this work, we propose to adapt this method for neonatal data to enable studies of early life tract development. For this purpose, a specific set of tools especially designed and tested for neonates were used2-4 to create ‘five-tissue-type’ (5TT) file used in the ACT framework and an accurate parcellation of the brain. Finally, the tractography was optimized using LiFE5.METHODS:
Image acquisition. 30 infants with mean age at birth 39+5 weeks (mean birth weight 3.42kg) underwent MRI at mean age 42+2 weeks. A Siemens MAGNETOM Verio 3T MRI clinical was used to acquire: 3D T1-weighted (T1w) MPRAGE with voxel size = 1×1×1mm3; diffusion MRI (dMRI) using a protocol consisting of 11 T2- and 64 diffusion-weighted (b = 750 s/mm2) single-shot, spin-echo, EPI volumes acquired with 2mm3 isotropic voxels.
Processing: sMRI data, brain extraction from T1w images using ALFA2, followed by a bias field using N46. dMRI data, Step 1: up-sampling by a factor of 2 before eddy current correction7; step 2: propagating the brain mask from the T1w space to the diffusion space using non-rigid registration8, 9; step 3: bias field correction for each dMRI dataset6. EPI distortion correction and alignment: First the T1w image was co-registered to the B0 image, then the B0 image was non-rigidly registered to the T1w volume9, while restricting the deformation only to the phase encoding direction11. Finally the computed transformation was applied to the dMRI volumes. To correctly align both modalities, the previously co-registered T1w volume was non-rigidly registered to the EPI corrected B0 volume. Tissue segmentation and anatomical parcellation: To parcellate each subject, the ENA33 atlas3 was registered to the T1w volumes of each subject. For the tissue segmentation, a neonatal-specific tissue segmentation method was used4. Specifically, a number of manually labeled images (k=10) ’uniformly’ distributed in the low-dimensional data space were used as the training data, and the tissue classification was performed using a machine learning based label fusion technique. Using the output of the segmentation algorithm, tissue probability maps were constructed and merged into a single 4D file (5TT) that can be used to create the WM/GM interface. Finally, the tractography, connectome and Track-Density Imaging (TDI) maps were created using MrTrix12-14 with different seeding strategies: 1) from FA > 0.15 and stopping at 0.1; 2) from the WM mask and 3) from the WM/GM interface using deterministic15 and probabilistic algorithms16. For visual inspection, the “effective” TDI was defined as the TDI created from the tractography that results after removing all the tracts with at least one termination outside the parcellation of the subject. Optimizing the tractography output: LiFE5 was applied with the aim of removing redundant connections. Connectivity measures: To compute connectivity parameters, the Brain Connectivity Toolbox and Network-Based Statistics (NBS) were used17, 18.
RESULTS:
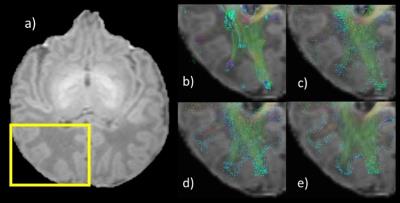
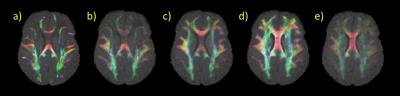
ACT combined with the GM/WM interface seeding (Fig. 1e) improves tractography by ending the tracts exclusively at the WM/GM interface, and not inside the GM (Fig. 1 b and c) or within WM (Fig. 1 b, c and d). Also ACT+GM/WM interface seeding provides a better coverage of the WM, as it is shown in Fig. 2, that shows the “effective” TDI for all different methods.
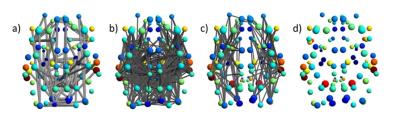
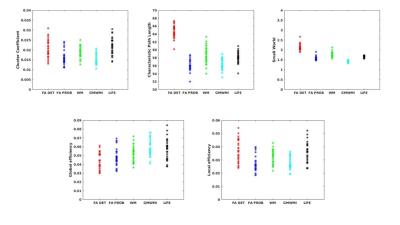
NBS was used to investigate the effect of each seeding strategy compared to ACT (Fig 3a-c), and the effect of LiFE on ACT is shown (Fig 3d). There were significant effects of seeding strategy, but there were no significant differences before and after applying LiFE to ACT (Figure 3d). For calculated global measures (Cluster Coefficient, Characteristic Path Length, Small-World, Global efficiency and Local Efficiency) the effect of the seeding strategy and LiFE was assessed using t-test, and Bonferroni adjusted p-value < 0.0125 was considered significant (Fig. 4).
DISCUSSION AND CONCLUSION:
The ACT method provides anatomically accurate coverage of WM in the neonate (Fig. 2 a, b and c). This affects measures extracted from the connectivity network, for example NBS (Fig. 3 a, b and c) and global network measures (Fig. 4). LiFE also affects the global network measures (Fig. 4)19.
In conclusion, the application of the ACT framework plus a refinement step (i.e. LiFE) has been applied to neonatal data for the first time, and it is shown to improve coverage of WM tracts compared with standard methods. The results suggest that ACT+LiFE may add value to neonatal connectivity studies.
Acknowledgements
We are grateful to the families who consented to take part in the study and to the nursing and radiography staff at the Clinical Research Imaging Centre, University of Edinburgh (http://www.cric.ed.ac.uk) who participated in scanning the infants. The study was supported by Theirworld, NHS Research Scotland, and NHS Lothian Research and Development. We thank Thorsten Feiweier at Siemens Healthcare for collaborating with dMRI acquisitions (Works-in-Progress Package for Advanced EPI Diffusion Imaging).References
1. Smith RE, Tournier J-D, Calamante F, Connelly A. Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage. 2012;62(3):1924-38.
2. Serag A, Blesa M, Moore EJ, Pataky R, Sparrow SA, Wilkinson AG, et al. Accurate Learning with Few Atlases (ALFA): an algorithm for MRI neonatal brain extraction and comparison with 11 publicly available methods. Scientific Reports. 2016;6:23470.
3. Blesa M, Serag A, Wilkinson AG, Anblagan D, Telford EJ, Pataky R, et al. Parcellation of the healthy neonatal brain into 107 regions using atlas propagation through intermediate time points in childhood. Frontiers in Neuroscience. 2016;10.
4. Serag A, Boardman JP, Wilkinson AG, Macnaught G, Semple SI, editors. A sparsity-based atlas selection technique for multiple-atlas segmentation: Application to neonatal brain labeling. 2016 24th Signal Processing and Communication Application Conference (SIU); 2016 16-19 May 2016.
5. Pestilli F, Yeatman JD, Rokem A, Kay KN, Wandell BA. Evaluation and statistical inference for human connectomes. Nat Meth. 2014;11(10):1058-63.
6. Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE transactions on medical imaging. 2010;29(6):1310-20.
7. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-90.
8. Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. Medical Imaging, IEEE Transactions on. 1999;18(8):712-21.
9. Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, et al. Fast free-form deformation using graphics processing units. Computer Methods and Programs in Biomedicine. 2010;98(3):278-84.
10. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26-41.
11. Wu M, Chang LC, Walker L, Lemaitre H, Barnett AS, Marenco S, et al. Comparison of EPI Distortion Correction Methods in Diffusion Tensor MRI Using a Novel Framework. Medical image computing and computer-assisted intervention : MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention. 2008;11(Pt 2):321-9.
12. Tournier JD, Calamante F, Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology. 2012;22(1):53-66.
13. Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35(4):1459-72.
14. Calamante F, Tournier J-D, Jackson GD, Connelly A. Track-density imaging (TDI): Super-resolution white matter imaging using whole-brain track-density mapping. NeuroImage. 2010;53(4):1233-43.
15. Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine. 2000;44(4):625-32.
16. Tournier JD, Calamante F, Connelly A, editors. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Intl Soc Mag Reson Med (ISMRM); 2010; Stockholm.
17. Zalesky A, Fornito A, Bullmore ET. Network-based statistic: Identifying differences in brain networks. NeuroImage. 2010;53(4):1197-207.
18. Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 2010;52(3):1059-69.
19. Qi S, Meesters S, Nicolay K, ter Haar Romeny BM, Ossenblok P. Structural brain network: What is the effect of LiFE optimization of whole brain tractography? Frontiers in Computational Neuroscience. 2016;10.
Figures