0795
Spatial and Temporal Relationship between Microstructural and Morphological Abnormalities of Alzheimer’s disease: Evidence in Cortical and Deep Gray MatterNan-Jie Gong1,2, Chun-Chung Chan3, Lam-Ming Leung3, Chun-Sing Wong4, Russell Dibb5, and Chunlei Liu5,6
1University of California Berkeley, Berkeley, CA, United States, 2Brain Imaging and Analysis Center, Duke University School of Medicine, Durham, NC, United States, 3United Christian Hospital, Hong Kong, 4The University of Hong Kong, Hong Kong, 5Duke University School of Medicine, 6Electrical Engineering and Computer Sciences, University of California Berkeley, Berkeley, CA, United States
Synopsis
Non-Gaussian diffusion metrics such as MK from DKI can complement conventional MD and FA for detecting microstructural changes, especially in deep gray matter. This can potentially improve the efficacy of diffusion metrics for serving as diagnostic imaging biomarkers. We also provided evidence supporting the proposed notion that microstructural changes in cortical and deep gray matter predate macrostructural changes such as volume and cortical thickness.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease and is the most prevalent type of dementia in the elderly. The first objective of the current study is to use non-Gaussian diffusion imaging for capturing microstructural abnormalities of AD and mild cognitive impairment (MCI) in the cortical and deep gray matter. We hypothesized that kurtosis metrics such as MK may reflect microstructural changes beyond those observed using conventional metrics such as MD and FA, thereby serving as a complementary imaging biomarker for early diagnosis and cognitive assessment of the disease. To investigate the spatial and temporal relationship between micro- and macrostructural changes, we also compared DKI metrics against thickness and volume in cortical and deep gray matter, respectively. We proposed that microstructural changes predated macrostructural changes and that the large-scale morphological changes may counterbalance the effect of alterations in microstructural compositions reflected by diffusion MRI.Materials and Methods
A cohort of 18 patients with Alzheimer’s disease (AD), 18 amnestic mild cognitive impairment (MCI) and 18 normal controls underwent morphological and DKI MRI. Images were investigated using regions-of-interest-based analyses of deep gray matter and voxel-based analyses of cortical gray matter using FreeSurfer.Results
In deep gray matter, DKI parametric abnormalities were observed in more regions than atrophy at the early MCI stage. Mean kurtosis (MK) exhibited the largest number of significant abnormalities among all DKI metrics including the bilateral hippocampus, thalamus, putamen and globus pallidus. At the later AD stage, diffusional abnormalities were found in fewer regions than atrophies (Figure 1). In cortical gray matter, abnormalities in thickness were mainly in the medial and lateral temporal lobes, which fit to the location of known early pathological changes. Microstructural abnormalities were predominantly in the parietal and even frontal lobes, which fit to the location of known late pathological changes (Figure 2 & Figure 3).Discussion
The results may be a reflection of a counterbalancing effect between a small-scale loss of microstructural compartments and a large-scale shrinkage of the whole nucleus. The volume of a nucleus represents a host of cytoarchitectural features including neuronal cell bodies, axons, dendrites, synapses and glia. At the initial phase, loss of cell bodies and disintegration of axons led to loss of microstructural complexity and increase in extracellular free diffusion space. These microstructural changes further manifested as increase in MD, decrease in MK, as well as probable decreases in FA. With the progression of disease, large-scale loss of neuronal complexity results in shrinkage that will later condensed the nucleus structure and recovered the density of neuronal compartments, thus ‘concealing’ the effect of loss of microstructural compartments initially captured by diffusion metrics (Figure 4). Although accumulations of beta-amyloid and ferritin might potentially increase MK in deep gray matter, they could be counterbalanced by local loss of neuronal structures and thus not observed in the present study (1-3). A contrary argument would be that the volume decreases predated microstructural changes; highly condensed space led to loss of neuronal compartments (Figure 4). If this assumption holds true, more widespread decreases in volume, but not changes in DKI metrics, is expected at the early aMCI stage. Regions exhibiting volume loss at the early aMCI stage are projected to exhibit abnormalities in DKI metrics at the later AD stage. Moreover, shrinkage of extracellular free diffusion space should result in a decrease in MD, increase in MK and probable increase in FA at the aMCI stage (Figure 4). All these postulated observations, especially the increases in MD, contradict the current study and, to the best of our knowledge, most of the previous DTI studies (4).Conclusion
Non-Gaussian diffusion metrics such as MK from DKI can complement conventional MD and FA for detecting microstructural changes, especially in deep gray matter. This can potentially improve the efficacy of diffusion metrics for serving as diagnostic imaging biomarkers. We also provided evidence supporting the proposed notion that microstructural changes in cortical and deep gray matter predate macrostructural changes such as volume and cortical thickness. These results not only deepened our understanding of neurodegenerative mechanisms but also can inform future diagnosis and the potential design of effective therapeutics by capturing subtle pathological changes at early phases of the disease, and predict regions in danger of atrophy as well as monitoring decline and recovery of cognitive functions.Acknowledgements
No acknowledgement found.References
1. Vanhoutte G, Pereson S, Delgado YPR, et al. Diffusion kurtosis imaging to detect amyloidosis in an APP/PS1 mouse model for Alzheimer's disease. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2013;69(4):1115-21. 2. Gong NJ, Wong CS, Hui ES, Chan CC, Leung LM. Hemisphere, gender and age-related effects on iron deposition in deep gray matter revealed by quantitative susceptibility mapping. NMR Biomed. 2015;28(10):1267-74. 3. Gong NJ, Wong CS, Chan CC, Leung LM, Chu YC. Aging in deep gray matter and white matter revealed by diffusional kurtosis imaging. Neurobiol Aging. 2014;35(10):2203-16. 4. Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2011;32(12):2322 e5-18.Figures
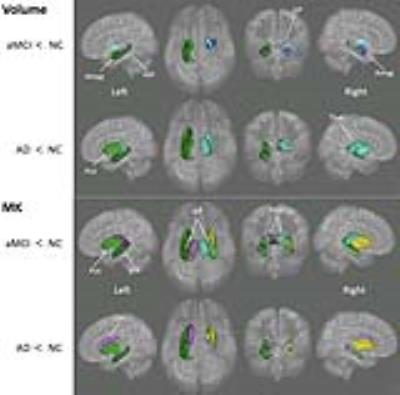
Figure 1
Deep gray matter
regions that showed significant differences in volume and MK between NC and aMCI/AD.
GP, globus
pallidus; Put, putamen; Thal, thalamus; HIP, hippocampus; Cau, Caudate nucleus;
Amyg, amygdala.
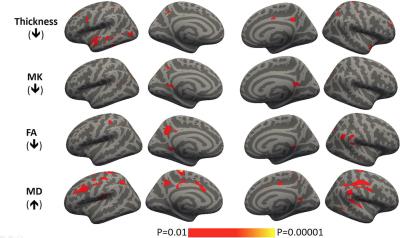
Figure 2 Cortex based spatial
statistics showing regions with significant abnormalities in thickness and DKI
metrics in aMCI as compared to NC.
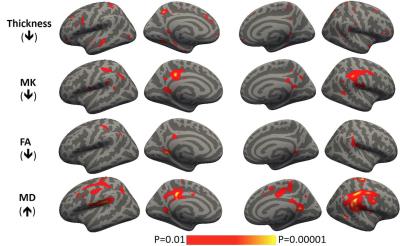
Figure 3 Cortex based spatial
statistics showing regions with significant abnormalities in thickness and DKI
metrics in AD as compared to NC.
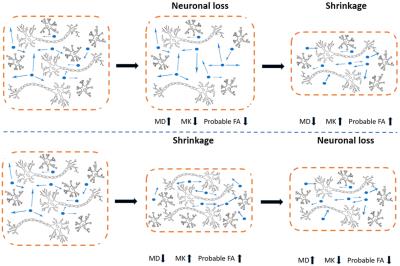
Figure 4 Two possible
temporal sequences between microstructural and morphological changes in gray
matter regions.