0794
A quantitative MRI study of APOE-dependent microstructural differences in young healthy volunteers using NODDI, qMT and g-ratio1CISC, BSMS, Brighton, United Kingdom, 2Psychology, University of Sussex, Brighton, United Kingdom
Synopsis
We present the first NODDI, qMT and g-ratio study of microstructural differences between carriers and non-carriers of the APOE-e4 gene. This gene is a risk factor for the development of Alheimer's Disease later in life. Our work shows that the more specific microstructural measures offered by the NODDI technique has revealed an increase in the non-tissue (Viso) component in the brain among APOE-e4 carriers. Importantly, the other NODDI parameters and g-ratio show no difference suggesting that although the tissue component among APOE-e4 carriers is reduced, there is no detectable difference to the underlying microstructure. This important finding has not been shown before, and supports the large body of evidence that shows young healthy APOE-e4 carriers are not disadvantaged across a number of cognitive domains and (in some tasks) out-perform their non-e4 peers.
Introduction
The presence of the APOE-e4 gene is the best known genetic risk factor for developing late-onset Alzheimer’s disease (AD)1. However, behavioural studies have shown that young carriers of this gene (e4+) may actually be advantaged in certain cognitive domains when compared to non-carriers (e4-)2,3. To study whether there is an effect of APOE-e4 on the brain microstructure of healthy young people we collected advanced MR imaging data that can probe the microstructure, particularly of the white matter. Specifically, we collected neurite orientation and dispersion imaging (NODDI) data4, that provides a means of detecting microstructural properties of dendrites and axons; quantitative magnetization transfer (qMT) that provides information about the macromolecular (myelin) component of tissue e.g. bound proton fraction, F. Myelin thickness was quantified using the g-ratio (the ratio of the inner to the outer diameter of a myelinated axon)5.Methods
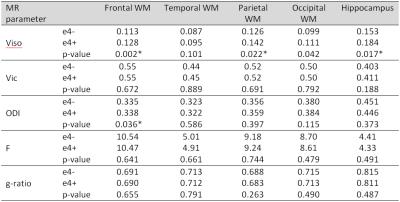
Paritcipants: 55 healthy young individuals, 27 e4- (carrying a double APOE-e3 gene) and 28 e4+ (carrying at least one APOE-e4 gene). Scanner: Siemens Avanto 1.5T. NODDI acquisition parameters: TR/TE=8400/99ms, 60 slices, matrix=96x96, FOV=240x240mm2, voxel-size=2.5x2.5x2.5mm3, three diffusion-weighted shells: b=300, 800, 2400s/mm2 with 9, 30, 60 directions respectively and 11 non-diffusion-weighted (b=0) volumes. Data analyzed using the NODDI toolbox for MATLAB (http://www.nitrc.org/projects/noddi_toolbox) to yield maps of isotropic volume fraction Viso, intracellular volume fraction Vic, and orientation dispersion index ODI. qMT acquisition parameters: bSSFP MT: 13 volumes were acquired using the bSSFP (MT parameters suggested by Gloor et al6). Matrix=192x256, FoV=180x240mm2, slice thickness=5mm; acquisition time=11min. T1-mapping was performed by acquiring three 3D GRE volumes with excitation flip angles 5°, 15°, 25°, TR/TE=30/5ms (positioning, matrix, FOV as qMT); acquisition time 3min. MT and T1-mapping data were coregistered and a Levenburg-Marquardt approach used to fit the qMT model to obtain bound proton fraction F, MT forward rate constant kf and T2 of the free pool T2f6. G-ratio maps were computed from the Viso, Vic and F, according to the method of Stikov5. Advanced Normalization Tools (ANTs; http://stnava.github.io/ANTs/) was used to warp all parameter maps to MNI standard space (voxel size=2mm3). SPM12 was used to smooth the parameters maps with a Gaussian kernel (full-width-half-maximum=6mm). Parameter maps were entered into a factorial design to identify the location of statistically-significant differences between e4+ and e4- genotype groups. A statistical threshold of p<0.05 family-wise error (FWE) corrected was used to assess significance. A secondary region-of-interest analysis was performed and mean (unsmoothed) values for ODI, Vic, Viso, F and g-ratio were extracted from the hippocampus and frontal, parietal, occipital and temporal lobes using the masks generated from the Talairach atlas provided by FSL. Statistical comparisons were made using a one-way ANOVA in SPSS version 22. Tract-based spatial statistics (part of FSL; FMRIB Software Library v5.0; http://fsl.fmrib.ox.ac.uk/fsl) was used to analyze differences in white matter tracts.Results
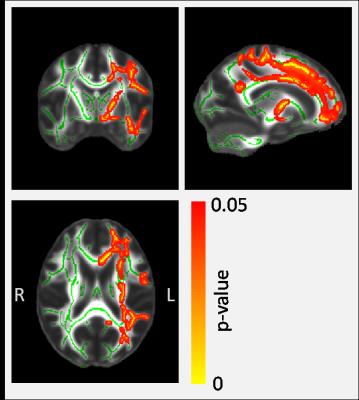
The data show greater Viso (larger non-tissue component) among the e4+ participants. This increase is widespread across multiple regions of the brain including frontal and parietal white matter and hippocampus (Table 1). A tract-based approach reveals that the changes are predominantly in the left hemisphere (Figure 1) and there were no locations where e4+ had lower Viso. There were no significant genotype-driven changes in Vic, ODI, F and g-ratio for any of the regions studied (Table 1), nor any tract-based differences.Discussion and Conclusions
This is the first study of the effects of APOE status on brain microstructure using NODDI and g-ratio measures. These relatively new MR techniques provide a number of specific microstructural properties that have not been investigated before in combination with APOE status. The study has found that healthy young people carrying the APOE-e4 gene (and who have an increased risk of developing AD in old age) exhibit a larger isotropic (non-tissue) component. Although this effect is widespread across the brain, significant differences were observed in the hippocampus and a tract-based analysis revealed a predominantly left-sided effect. Importantly, there were no complementary differences observed with the other NODDI parameters or with qMT and g-ratio, suggesting that although the tissue component among APOE-e4 carriers is reduced, there is no detectable difference to the underlying microstructure of the tissue. This observation supports the large body of evidence showing young healthy APOE-e4 carriers are not disadvantaged across a number of cognitive tasks and (in some domains) actually out-perform their non-e4 peers. It is intriguing to speculate that an increased non-tissue component (i.e. a smaller tissue component) such as that identified here, may play an important role in the development of AD and brain atrophy among the e4+ later in life.Acknowledgements
No acknowledgement found.References
(1). Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA.Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families.Science. 1993;261(5123):921-3.
(2). Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Boesiger P, Hock C, Nitsch RM, Papassotiropoulos A, Henke K. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex. 2007;17(8):1934-47
(3). Rusted JM, Evans SL, King
SL, Dowell NG, Tabet N, Tofts PS. APOE e4 polymorphism in young
adults is associated with improved attention and indexed by distinct
neural signatures. Neuroimage. 2013;65:364–373.
(4). Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–1016.
(5) Stikov N, Campbell JSW., et al. In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage 2015;118:397-405.
(6). Gloor M, Scheffler K, Bieri O. Quantitative magnetization transfer imaging using balanced SSFP. Magn Reson Med. 2008;60(3):691-700.
Figures