0791
Phase imaging of axonal integrity of cranial corticospinal tract in experimental spinal cord injury at 9.4T1High Magnetic Field Lab of Chinese Academy of Sciences, Hefei, People's Republic of China, 2Department of Neurology, Anhui Medical University, Hefei, People's Republic of China
Synopsis
Spinal cord injury (SCI) leads to neuronal cell death, axonal damage and demyelination. Brain undergo anatomical changes following SCI. Recently MR phase imaging has shown promising application in visualizing demyelination. In this study we explored the possibility to investigate integrity of cranial corticospinal tract in SCI using phase imaging. Diffusion tensor imaging was also used to verify the axonal integrity. The results showed phase contrast decreased along with axial diffusivity did not significantly change in contralateral pyramid two weeks post-injury compared to pre-injury levels. Thus, phase imaging is a potential endogenous biomarker for brain axonal integrity after SCI.
INTRODUCTION
Spinal cord injury (SCI) leads to neuronal cell death, axonal damage and demyelination. Both spinal cord 1 and brain 2 undergo anatomical changes following SCI. Recently MR phase imaging has shown promising application in visualizing demyelination 3 or axonal damage 4. Therefore, in this study we explored the possibility to investigate integrity of cranial corticospinal tract (CST) in SCI using phase imaging. Diffusion tensor imaging (DTI), and its metrics, e.g. axial diffusivity (λ∥) were also used to verify the axonal integrity and preclude the potential contribution of axonal injury to the observed decrease in frequency contrast. We also correlated the MRI findings to immunohistochemistry.MATERIALS and METHODS
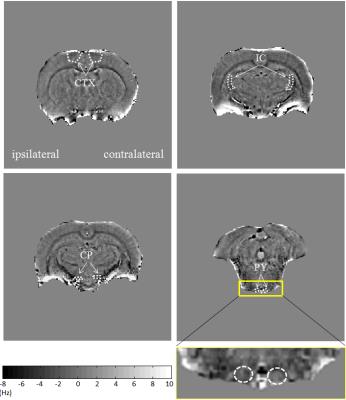
These studies were performed on eight Sprague-Dawley rats weighing from 300–350 g. A T9 spinal cord hemisection was performed as described previously 5. MR scans were carried out on day 0 (pre-injury) and 2 weeks post-injury. All MR studies were performed on an Agilent 9.4 T/400 mm spectrometer. For in vivo MR studies, rats were anesthetized with a mixture of 2% isoflurane, 30% oxygen and air. A standard 2D gradient echo sequence was used to acquire the phase images with TR/TE = 1500/15 ms, flip angle = 60o, matrix size 192 × 192, FOV = 25 mm × 25 mm, 48 slices with slice thickness of 0.5 mm, a spectral width of 10'000 Hz, 2 averages and a total scan time 9 minutes and 36 seconds. Diffusions images were acquired using a multi-shot spin-echo echo planar imaging (EPI) sequence with TR/TE = 3000/24 ms, 8 shots, matrix size 128 × 128, FOV = 25 mm × 25 mm, 24 slices with slice thickness = 1 mm, 2 averages. A total of thirty diffusion encoding directions were used at a b-value of 800 s/mm2. Phase images were obtained as described previously 6. The DTI metrics, i.e. λ∥ and radial diffusivity (λ⊥) were then calculated using several steps within the FSL 5.0. To quantify CST changes, each MR dataset was analyzed using ImageJ. Statistical comparisons made between the pre-injury and post-injury levels were carried out by two tailed Student’s unpaired t tests.RESULTS
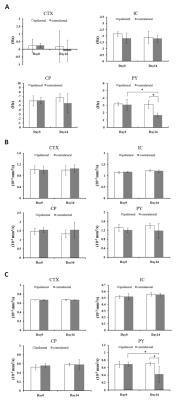
Fig.2 shows that the phase contrast decreased mainly in contralateral pyramid by 46% two weeks post-injury compared to pre-injury levels. Significant reduction of 39% in contralateral pyramid (p < 0.05) two weeks post-injury compared to pre-injury levels when quantified λ⊥ values. The λ∥ in all those regions did not significantly change 2 weeks post-injury compared to pre-injury levels.DISCUSSION
The reduction of frequency contrast along with no significant changed λ∥ were observed in the contralateral pyramids in this study, suggestive of loss of myelin or demyelination without obvious axonal damage in the CST white matter. These MRI findings were confirmed by immunohistological results, i.e. myelin basic protein staining lost but without obvious reduction in neurofilament staining after two weeks after injury. The reduced λ⊥in the contralateral pyramids, which may be associated with activated astrocytes according to the increased expression of GFAP in those regions. In conclusion, phase imaging is a potential endogenous biomarker for brain axonal integrity after spinal cord injury.Acknowledgements
This work was supported by the National Natural Science Foundation of China (http://www.nsfc.gov.cn/) grant 81201068.References
1. Cohen-Adad J, El Mendili MM, Lehericy S, Pradat PF, Blancho S, Rossignol S, et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55(3):1024-33.
2. Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, et al. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19(1):224-32.
3. Liu C, Li W, Johnson GA, Wu B. High-field (9.4 T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. Neuroimage. 2011;56(3):930-8.
4. Yablonskiy DA, Luo J, Sukstanskii AL, Iyer A, Cross AH. Biophysical mechanisms of MRI signal frequency contrast in multiple sclerosis. Proc Natl Acad Sci U S A. 2012;109(35):14212-7.
5. Herrera JJ, Chacko T, Narayana PA. Histological correlation of diffusion tensor imaging metrics in experimental spinal cord injury. J Neurosci Res. 2008;86(2):443-7.
6. Marques JP, Maddage R, Mlynarik V, Gruetter R. On the origin of the MR image phase contrast: an in vivo MR microscopy study of the rat brain at 14.1 T. Neuroimage. 2009;46(2):345-52.
Figures