0787
Diffusion imaging in grade II and III gliomas depends upon new vs recurrent status and enhancing vs nonenhancing status.1University of California San Francisco, San Francisco, CA, United States
Synopsis
The relationship of diffusion imaging parameters with prognostic histological and molecular factors for patients with grade II and III gliomas is unclear, particularly for tumors that are non-enhancing on post-Gadolinium images. We investigated the relationship of ADC and FA values with histological tumor score, tumor grade, and molecular characteristics for non-enhancing (NE) vs contrast-enhancing (CE) and newly-diagnosed (ND) vs recurrent (REC) disease. In NE patients, histopathological and molecular characteristics associated with poorer clinical outcome were found to have higher ADC and lower FA. In CE patients, some characteristics associated with poorer outcome hadlower ADC and higher FA.
Purpose
Treatment planning for patients with grade II and grade III glioma is challenging, and there is no consensus on the timing of radiation and chemotherapy. Decisions are based upon histopathological and molecular characteristics of resected tissue, in combination with MRI findings. Obtaining tissue samples from regions that reflect the most malignant tumor characteristics is essential for optimal treatment planning. Diffusion imaging can be used to assist in identifying target locations for tissue sampling, especially in non-enhancing gliomas. Estimates of the Apparent Diffusion Coefficient (ADC) and Fractional Anisotropy (FA) have been associated with the degree of abnormal tissue microstructure. In WHO grade IV glioma, regions of low ADC and high FA are reliably indicative of malignant behavior1-3. But in grade II and III gliomas, the relationship between diffusion imaging parameters and prognostic histological and molecular factors is unclear, particularly in non-enhancing tumors. In the current study, we investigated the relationship between diffusion imaging and histological tumor score, tumor grade, and molecular characteristics for non-enhancing (NE) vs contrast-enhancing (CE) and newly-diagnosed (ND) vs recurrent (REC) disease.Methods
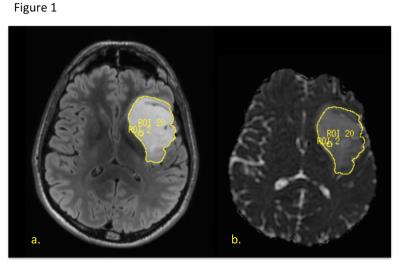
285 patients underwent a pre-surgical 3T GE MR exam using an 8 channel head coil. The MRI examination included T1-weighted IRSPGR, T2-weighted 3D FSE and/or XETA T2 FLAIR, and 6 directional axial Diffusion Weighted Imaging with b=1000s/mm2. During surgery, the locations of tissue samples were recorded relative to the FSE image with a surgical navigation system. WHO 2016 diagnosis, grade, MIB-1/ki-67, tumor score (based on contribution of tumor cellularity to total cellularity), IDH mutation status, and co-deletion of 1p19q were determined by standard pathology, immunohistochemistry and FISH. 5mm spherical ROIs centered at the tissue sample locations were used to calculate the median ADC and FA values around each location, normalized to normal brain tissue. The nADC (normalized ADC) and nFA (normalized FA) values were also calculated for the entire T2 Flair lesion, and in CE gliomas, the contrast-enhancing T1 lesion. The effects of histological and molecular tumor characteristics on diffusion parameters were evaluated according to enhancement and recurrence status using repeated-measure analysis of variance.Results
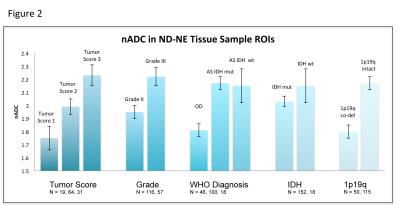
In ND-NE patients, 185 tissue samples were obtained from 78 patients. Tissue samples with higher nADC and lower nFA values had worse tumor scores, higher MIB scores, and came from tumors with higher WHO grade, astrocytoma diagnosis, intact 1p19, and IDH wildtype. In the entire T2 flair lesion, IDH wildtype patients had higher nADC than IDH mutant patients, and 1p19q intact patients had higher nADC than co-deleted patients. No tissue samples were obtained from contrast-enhancing locations in ND patients with contrast enhancement.
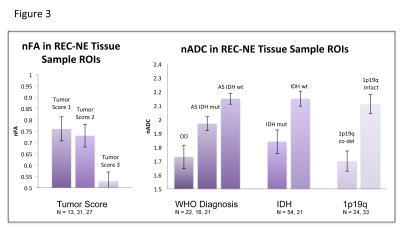
In REC-NE patients, 72 tissue samples were obtained from 38 patients. Tissue samples with lower nFA values had better tumor scores, and tissue samples with higher nADC values had lower MIB scores, and were from tumors with astrocytoma diagnosis, intact 1p19q, and IDH wildtype. In the entire T2 flair lesion, IDH wildtype patients had higher nADC than IDH mutant patients. Across all patients, nFA was lower for ND-NE than REC-NE patients.
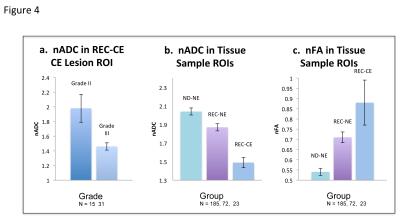
In REC-CE patients, 23 tissue samples in contrast enhancing regions were obtained from 16 patients. There were no significant diffusion MRI differences associated with histopathological or molecular characteristics. nADC was lower and nFA was higher for REC-CE than REC-NE tissue samples. In the entire contrast-enhancing lesion, grade III patients had lower nADC than grade II patients, and 1p19q co-deleted patients had higher nFA than 1p19q intact patients. In the entire T2 flair lesion, grade III patients had lower nADC than grade III patients. Across all patients, nADC was lower and nFA was higher for REC-CE than REC-NE T2 flair lesions.
Discussion
In NE lower grade glioma patients, especially ND, histopathological and molecular characteristics that are associated with poorer clinical outcome4 were found to have higher nADC and lower nFA. In CE patients, some histopathological and molecular characteristics associated with poorer clinical outcome were associated with lower nADC and higher nFA. In ND-NE patients, higher nADC and lower nFA may be driven by increased disruption of normal tissue architecture, whereas in CE patients, increased tumor cell density may drive lower nADC. These results suggest that when considering nADC and nFA values in tissue sample targeting and in evaluations of tumor malignancy and treatment response in grade II and III gliomas, the interpretation of these values depends upon the patient’s enhancement and recurrence status.Acknowledgements
This work was supported by NIH R01 CA159869 and NIH P50 CA097257.References
1.Khayal, IS, Vandenberg, SR, Smith, KJ et al. MRI apparent diffusion coefficient reflects histopathologic subtype, axonal disruption, and tumor fraction in diffuse-type grade II gliomas. Neuro-Oncology 13:1192-1201, 2011.
2. Wen Q, Jalilian L, Lupo JM et al. Comparison of ADC metrics and their association with outcome for patients with newly diagnosed glioblastoma being treated with radiation therapy, temozolomide, erlotinib and bevacizumab.Journal of Neuro-Oncology 121:331-339, 2015.
3. Togao, O., Hiwatashi, A., Yamashita, K. et al. Differentiation of high-grade and low-grade diffuse gliomas by intravoxel incoherent motion MR imaging. Neuro-Oncology 18:132-41, 2015.
4. Claus, EB, Walsh, KM, Wiencke, JK et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurgical Focus 38:1-10, 2015.
Figures