0782
Respiratory motion-corrected simultaneous cardiac PET and coronary MR angiography using a hybrid 3T PET-MR1Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare, Frimley, United Kingdom
Synopsis
Physiological motion remains a major challenge for cardiac PET-MR. Here we propose a framework for non-rigid respiratory motion-corrected simultaneous Coronary MR Angiography (CMRA) and cardiac PET. Motion estimated from low-resolution MR image navigators and from CMRA data itself is used for correcting CMRA and PET datasets to the same respiratory position. The proposed CMRA approach was validated in ten healthy subjects. Results from the PET-CMRA framework on three patients show that motion-corrected PET images have improved sharpness compared to uncorrected reconstructions, whereas motion-corrected CMRA images have improved coronary vessel length and sharpness compared to uncorrected and translational-corrected images.
Introduction
Whole-body PET-MR systems offer great potential for simultaneous assessment of myocardial viability by PET and coronary lumen integrity by coronary MR angiography (CMRA) in a single examination(1). However, image degradation due to motion remains a major challenge in both modalities. Current developments for motion-compensated PET-MR focus mainly on improving PET image quality by acquiring MR images for motion estimation only(2). This leads to long acquisition times since diagnostic MR images are acquired after the simultaneous PET-MR acquisition. Here we propose a novel acquisition and reconstruction scheme that allows for acquiring motion-corrected CMRA and cardiac PET in an efficient single examination. Motion is estimated from MR images, so that 2D translational and 3D non-rigid motion-corrected CMRA can be performed efficiently (no data rejection, shorter scan time, simplified planning) on a 3T PET-MR scanner, while simultaneously providing non-rigid motion fields for MR-based PET respiratory motion correction.Methods
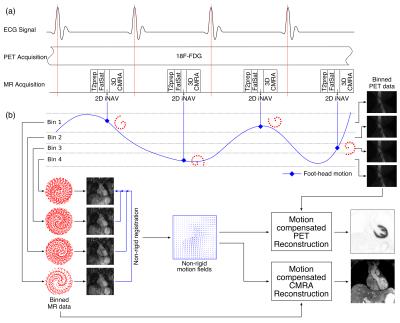
PET-MR acquisition consists of an ECG-triggered free-breathing CMRA sequence simultaneously acquired with cardiac list mode PET data (Fig1a). CMRA data is acquired using a 3D T1-weighted spoiled gradient echo sequence with a fully sampled golden-step Cartesian spiral profile order sampling trajectory(3). A low-resolution 2D image navigator (iNAV)(4) is acquired at each cardiac cycle by spatially encoding low flip angle start-up echoes preceding the CMRA acquisition. Fat saturation and adiabatic T2 preparation pulses are performed to improve contrast between blood and myocardium. The iNAVs are used to estimate foot-head and right-left motion in a beat-to-beat fashion, providing translational motion estimates to correct the MR data. Foot-head motion is used to bin both the PET and MR data (Fig1b). 3D non-rigid motion between respiratory bins is estimated from MR images reconstructed at each respiratory position and is then used for correcting the CMRA (directly in the reconstruction(5)) and the emission and attenuation PET datasets to the same respiratory position.
Ten healthy subjects were scanned on a Biograph mMR scanner (Siemens Healthcare, Germany) using a prototype implementation of the proposed sequence (304x304x40-48 matrix size, 1x1x2mm3 resolution TR/TE=3.7/1.7ms, FA=15°, T2prep=50ms). A subject-specific trigger delay and acquisition window (89 to 119ms) were set coinciding mid-diastolic rest period. For the iNAV, 14 start-up echoes (same FOV as CMRA, FA=3º) were used. Additionally, a gated and tracked image (6mm gating window, tracking scaling factor=0.6) was acquired for comparison. The whole PET-MR framework was tested in three oncology patients (scanned 2.2 hours after injection of 319.7 MBq of 18F-FDG, on average), using the same acquisition parameters described for the healthy subjects. List-mode PET data was acquired during the whole CMRA acquisition (~10min).
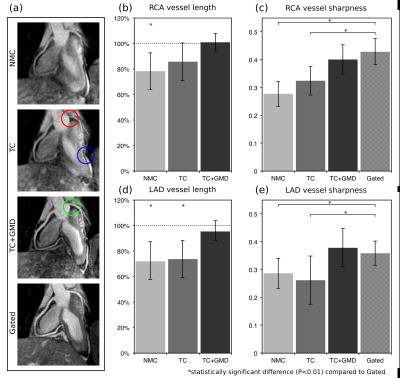
CMRA data was reconstructed with the proposed translation plus non-rigid motion correction (TC+GMD), 2D translational motion correction only (TC) and without motion correction (NMC). PET image reconstruction was performed in Siemens e7 Tools (OSEM, 3 iterations, 21 subsets, PSF modelling, 2.03x2.08x2.08mm3 resolution, 127x344x344 matrix size). Three reconstructions were performed for PET data: reconstruct-transform-average(6) motion-corrected (MC), uncorrected (NMC) and Gated reconstruction at end-expiration.
Results
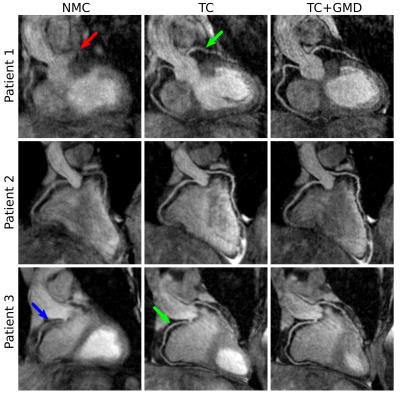
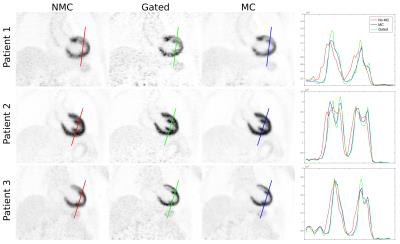
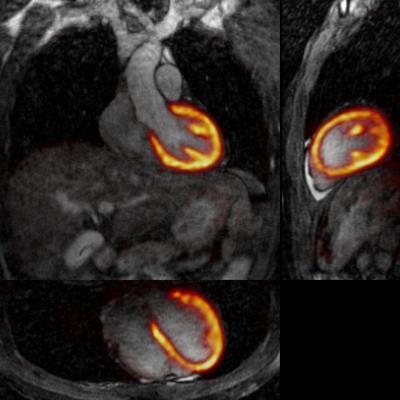
3D CMRA images were reformatted to visualize the left anterior descending (LAD) and right coronary artery (RCA) simultaneously, and vessel length and sharpness were computed for each coronary artery. For the healthy subjects, similar image quality was obtained for TC+GMD and Gated images (Fig2a), and metrics for the RCA and LAD show no statistically significant difference (P>0.01) between TC+GMD and Gated (Fig2b-e). For the patients, TC reconstructed images allow for visualising the proximal segments of each vessel, and further improvement can be observed when applying the proposed TC+GMD (Fig3). Reconstructed PET images were assessed with line profiles across the left ventricle, showing that MC improves sharpness of the myocardium compared to NMC while reducing noise compared to Gated (Fig4). Fig5 shows example slices for a representative patient of a fused motion-corrected PET-CMRA image.Conclusion
We present a novel framework for simultaneous coronary MR angiography and cardiac PET on a 3T PET-MR scanner. Our approach allows for non-rigid respiratory motion correction of both the MR and PET datasets, producing diagnostic images for simultaneous visualisation of the coronary arteries and myocardial viability. The motion-corrected CMRA framework at 3T was validated in healthy subjects. No statistically significant differences in image quality were found when comparing to a standard 1D diaphragmatic navigated and tracked acquisition, however, our approach has shorter and predictable scan time and provides non-rigid motion information for correcting PET data. The whole PET-MR framework was tested in three oncology patients, and results show increased sharpness and reduced image noise compared to uncorrected and gated PET reconstructions respectively. Future work includes validating our approach in patients with cardiac disease.Acknowledgements
The authors acknowledge financial support from: (1) EPSRC: EP/N009258/1, (2) King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1).References
1. Rischpler C et al. Hybrid PET/MR imaging of the heart: potential, initial experiences, and future prospects. J Nucl Med 2013;54:402–415.
2. Munoz C et al. MR-Based Cardiac and Respiratory Motion-Compensation Techniques for PET-MR Imaging. PET Clin 2016;11:179–191.
3. Prieto C et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J Magn Reson Imaging 2015;41:738–746.
4. Henningsson M et al. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med 2012;67:437–445.
5. Cruz G et al. Highly efficient nonrigid motion-corrected 3D whole-heart coronary vessel wall imaging. Magn Reson Med 2016. doi: 10.1002/mrm.26274.2.
6. Picard Y et al. Motion correction of PET images using multiple acquisition frames. IEEE Trans Med Imaging 1997;16:137–144.
Figures