0781
Motion correction on a human PET/MR scanner: Clinical feasibility of a motion correction system in patients – an update report1Department of Radiology, University of Tuebingen, Tuebingen, Germany, 2Institute of Signal Processing and System Theory, University of Stuttgart, Stuttgart, Germany, 3University of Stanford, Palo Alto, CA, United States, 4Section on Experimental Radiology, University of Tuebingen, Tuebingen, Germany, 5LaTIM, INSERM, University of Bretagne, Brest, France, 6Department of Electronic Engineering, Chinese University of Hong Kong, Hong Kong, Hong Kong
Synopsis
The diagnostic accuracy of Positron-Emission-Tomography/Magnetic Resonance (PET/MR) is often reduced in regions affected by respiratory and cardiac motion. These motion-induced artifacts can be corrected by an MR-derived motion model (MM). Here, we improved the previously presented PET/MR motion correction system by two new sampling trajectories for the MR motion imaging and extend it by the usage of an additional Compressed Sensing reconstruction (BART), an optical-flow based registration (LAP) and the incorporation of motion correction into a listmode-based PET reconstruction (CASToR) which are all integrated into the Gadgetron-based reconstruction pipeline for a clinical feasible setup. In-vivo patient data substantiated the improvements.
Purpose
In oncologic imaging, hybrid PET/MR scanners offer a great potential for improving diagnostic accuracy in body regions impaired by motion. In order to guarantee a diagnostic PET image quality, long examination times in the range of several minutes per bed position are required. Hence, inevitable respiration and cardiac motion degrade the resulting PET image. These motion artifacts manifest in the PET image as a smearing along the dominant moving direction. The simultaneously acquired MR can perform a motion imaging from which a retrospectively-derived motion model (MM) is applied to the PET image1,2,3. However, the additional MM sequence workload should be kept as low as possible to provide the flexibility of acquiring further diagnostic MR sequences which are usually performed during the PET scan. We proposed a 4D Cartesian MR acquisition4 which subsamples the phase-encoding directions subject to a Gaussian ESPReSSo subsampling mask5 under free-movement conditions to provide a high-resolution image within short acquisition time. The generation of a reliable MM requires a continuous respiratory and cardiac motion signal covering the complete PET examination time. We addressed this by a sensor fusion approach mapping simultaneously acquired surrogate markers (respiratory belt, electrocardiography-derived respiratory signal, camera) to the MR self-navigator6. To provide a clinical feasible workflow all reconstruction and correction steps were carried out online by streamling-processing on external workstation7 via Gadgetron8. In previous studies we showed the feasibility of this method and the improvements on the PET image quality for an image-based PET motion correction (MC)6,9,10. However, as previously reported4 for some subjects an increase in subsampling and eddy current related artifacts was observed which reduced the achievable image quality. To prevent this issue and further improve the MC system, we will address in this work three problems: a) reducing artifacts from subsampled MR acquisition by two new k-space trajectories based on a Cartesian grid with pseudo-radial/spiral trajectory and variable-density Poisson-Disc ESPReSSo subsampling, b) speeding up MR reconstruction using BART11 inside Gadgetron with the incorporation of an MC regularization9 based on an optical-flow registration (local all-pass;LAP)12 and c) improving PET image quality by CASToR13,14 as a listmode-based motion correction and reconstruction.Materials and Methods
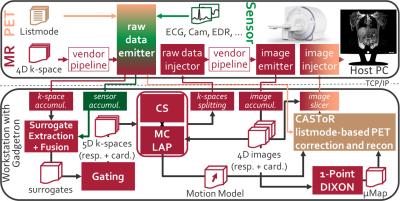
The complete PET/MR MC system for Gadgetron8 is shown in Fig.1.
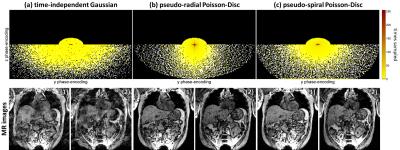
Acquisition: MR, PET and surrogate data are acquired simultaneously within the first minute of the examination. During the remaining PET acquisition time, other diagnostic MR sequences can be run. The 3D spoiled gradient-echo MR motion imaging sequence (TE=1.23ms,TR=2.6ms,FOV=500x500x360mm, matrixSize=256x256x144) is improved by employing a pseudo-radial or pseudo-spiral trajectory in ky/kz plane which is directly gridded in the acquisition to Cartesian space with golden angle increment between subsequent spokes and a variable-density Poisson-Disc subsampling within each spoke. This enables to image non-quadratic FOVs and to employ an ESPReSSo subsampling5, as shown in Fig. 2.
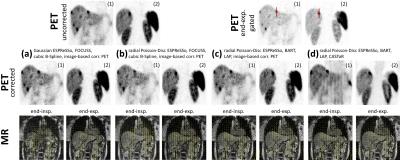
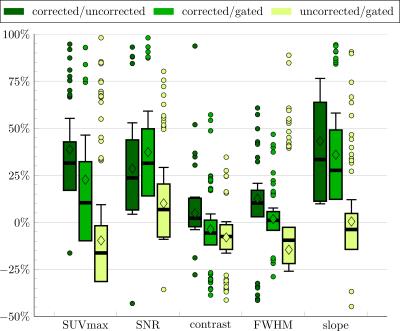
Reconstruction: All reconstructions steps are implemented into Gadgetron. The respective emitter and injector functors take care of the data streaming and conversion. The vendor reconstruction pipeline is kept intact to benefit from the vendor-specific correction methods (e.g. geometrical distortion correction). For the Compressed Sensing reconstruction, the BART toolbox11 is tested against the previously proposed FOCUSS algorithm6,10. Moreover, BART is extended by a motion-compensated regularization9 with an optical-flow based registration (LAP)12. The LAP algorithm provides faster and more accurate deformation fields12 which serve as MM input to the motion-corrected listmode-based PET reconstruction implemented via CASToR13,14. Coronal in-vivo patient data were acquired for 37 patients (20 female, age 60±9; 2 patients with new k-space trajectories) with suspected lung or liver metastasis on a 3T PET/MR (Biograph mMR, Siemens). A comparison of the different acquisition (Gaussian, pseudo-radial/spiral Poisson-Disc), correction (cubic B-spline registration, LAP) and reconstruction (FOCUSS, BART) methods of the MR and of the PET images (image-based and listmode-based correction) is illustrated. ROIs and lines were placed on lesions of the corrected, uncorrected and end-expiratory gated PET image to extract quantitative metrics.
Results and Conclusion
MR images recorded with the proposed sampling strategy show reduced artifacts due to the confined k-space trajectory (Fig.2). This trajectory also improved scan time efficiency (samples per motion state) and allows thus the acquisition of isotropic resolution without reducing image quality using the reported regime. A reliable MM could be reconstructed within ~8min compared to previously ~15min which was utilized in CASToR yielding an improved PET image quality in comparison to an image-based PET MC approach (Fig.3+4). These results are supported by the extracted PET metric values of moving lesions as percentage improvements (Fig.5). In conclusion, the clinical feasible PET/MR MC system was improved by the suggested extensions.
The MC system for PET/MR is publicly available:
https://sites.google.com/site/kspaceastronauts/motion-correction/pet-mr-motion-correction
Acknowledgements
The authors would like to thank Carsten Gröper and Gerd Zeger for data acquisition and Brigitte Gückel for study coordination. Sergios Gatidis and Ferdinand Seith are gratefully acknowledged for organisatory help in patient recruitment and reading.References
[1] Würslin
et al., JNM 2013;54. [2] Grimm et
al., Med Image Anal 2015;19(1). [3]
Manber et al., JNM 2015;56(6). [4] Küstner
et al., MRM 2016. [5] Küstner et al.,
IEEE TMI 2016. [6] Küstner et al., Proc ISMRM 2016. [7] Schwartz et al., Proc ISMRM 2016. [8] Hansen et al., MRM 2013;69(6). [9] Küstner et al., IEEE Proc ICASSP 2015. [10] Küstner et al., Proc ISMRM 2015. [11] Uecker et al., Proc ISMRM 2015. [12] Gilliam et al., IEEE Proc ISBI 2016. [13] Merlin et al., IEEE Proc NPSS 2015. [14] Merlin et al., www.castor-project.org.
Figures