0755
Development and Clinical Implementation of Very Light Weight and Highly Flexible AIR Technology Arrays1Radiology, Stanford University, Stanford, CA, United States, 2GE Healthcare, 3Electrical Engineering, Stanford University, 4Electrical Engineering and Computer Science, UC Berkeley
Synopsis
Pediatric body MRI faces challenges of widely varying patient sizes, heterogenous imaging indications, and limited patient cooperation. These difficulties are compounded by receiver array coils that are often mismatched to patients’ size. In this work, we develop a novel light-weight flexible coil array that can be placed on a patient, and combined with a high-density posterior array and determine feasibility of clinical use in a pediatric setting. The resulting coil is well received in the clinic and yields good image quality.
Purpose
Pediatric body MRI faces challenges of widely varying patient sizes, heterogenous imaging indications, and limited patient cooperation. These difficulties are compounded by receiver array coils that are often mismatched to patients’ size. In particular, the arrays can be large and thus physically intimidating to young non-sedated patients, while constricting respiration in sedated small children. Recently, there has been much movement away from traditional array architectures based upon copper strip arrays towards new technologies1 including printed conductive inks2, along with the use of new components like MEMs3, to offer a route to even lighter and more form-fitting receiver arrays. In this work we aim to develop a novel light-weight flexible coil array that can be placed on a patient, and combined with a high-density pediatric posterior array4 or conventional posterior phased array, and determine feasibility of clinical use in a pediatric setting.Materials and Methods
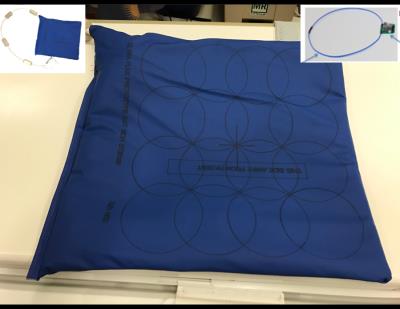
The prototype coil based upon AIR Technology, used in this study contains sixteen 11cm diameter loops and weighs approximately 480 grams without the external cable (see Fig. 1) facilitating a FOV of 33cm x 37cm. AIR Technology utilizes a custom process to minimize reactance and conductor loss to realize ultra-flexibility while minimizing weight. A custom noise controlling preamplifier is the center piece of the minimalistic electronics which allows element overlap beyond the historic “critical overlap” regime5 (Fig 1 inset). Packaging materials were selected on technical fabrics for low weight, flexibility, and safety. The conductor and electronics combination controls loop interactions and allows coil overlaps the freedom to reposition as required without significant performance degradation. Packaged accordingly, this results in high performance arrays with extremely low weight and flexibility. The coil was made compatible with a high density custom pediatric posterior array1 and a standard adult posterior array (Neocoil 32ch Torso).
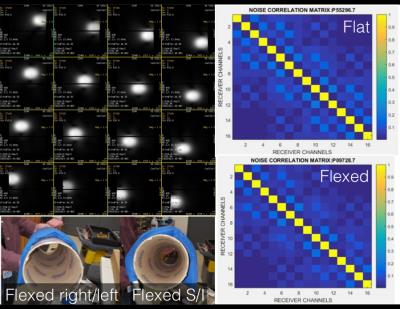
Element decoupling was assessed in both a flat and a highly flexed configuration. With IRB approval and informed consent/assent, 18 consecutive patients ages 10 and under referred for 3T pediatric MRI were recruited. MR technologists, nurses, and anesthesiologists were surveyed to determine preference for the flexible array compared to conventional coils. Routine imaging protocol was completed, and diagnostic adequacy of images assessed.
Results
Phantom testing demonstrated the elements were highly decoupled from each other (<-20db between channels) as seen in Fig 2. Measured element Qunloaded / Qloaded ratios were approximately 4.5. The coil demonstrated good flexibility in all directions (Fig 2). Noise correlation data was collected both with the array flat and flexed around cylinder with no deterioration found (Fig 2).
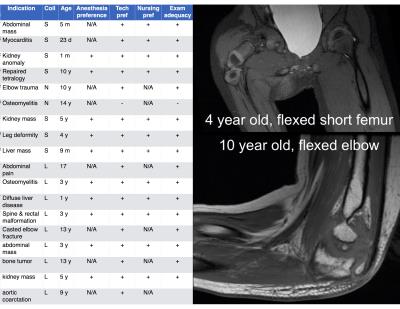
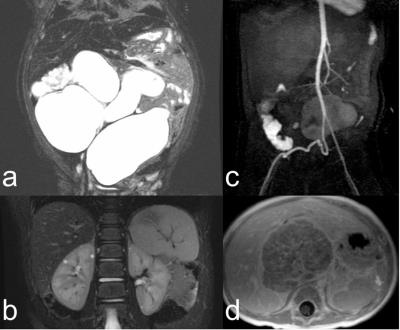
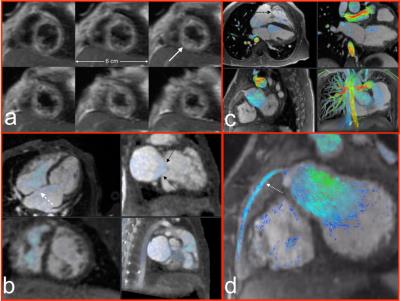
Recruited patient indications are shown in Figure 3 (left), spanning musculoskeletal, abdominal, and cardiovascular exams. Anesthesiologist and nurse preference were uniformly for the flexible coil (10/10 and 12/12 cases). Technologist preference for flexible coil was present in 94% of cases, and those cases were diagnostically adequate. In one case, coil coverage for long bones of the leg was not adequate. In no case was the coil offset from the patient with bolsters due to concern of the weight of the coil restricting respiration. Representative images are shown in Figures 3 – 5, which show outstanding image quality.
Conclusion
Construction of a highly flexible close fitting coil array that is light is feasible. Element to element decoupling was found to be highly robust to the coil wrapping or flexing. The coil is likely to be preferred by anesthesiologists and pediatric nurses, and yield diagnostically adequate images.Acknowledgements
We gratefully acknowledge support of NIH R01EB019241.References
1. “Redefining the World of Coils with MEMS Technology” GE Signa Pulse: Academic Issue, 2015
2. “Screen-printed flexible MRI receive coils” by Corea et al.: Nature Communications 7, Article number: 10839 (2016)
3. “Custom MEMs Switch for MR Surface Coil decoupling” by Spence et al. #0704 Proc Intl Soc Mag Reson Med 23, 2015.
4. “A Semi-flexible 64-channel receive-only phased array for pediatric body MRI at 3T” by Zhang et al., Magn Reson Med. 2015 Sep;76(3):1015-21.
5. “The NMR Phased Array” by Roemer et al. Magn Reson Med. 1990 Nov;16(2):192-225.
Figures