0754
Mapping of Abnormal Aortic Hemodynamics in 515 Patients with Aortopathy1Radiology, Academic Medical Center, Amsterdam, Netherlands, 2Radiology, Northwestern University, Chicago, IL, United States, 3Biomedical Engineering, Northwestern University, Chicago, IL, United States, 4Division of Surgery-Cardiac Surgery, Northwestern University, Chicago, IL, United States, 5Medicine-Cardiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 6Cardiac Sciences, University of Calgary, Calgary, Canada
Synopsis
4D flow MRI-derived 3D velocity and wall shear stress (WSS) maps in a large cohort of patients with aortopathy (n=515), stratified for valve morphology and stenosis severity, were compared with age-matched cohort-averaged maps of healthy controls (n=56) to yield maps of abnormally elevated hemodynamics. These maps were projected onto shared geometries and summed to map the incidence of abnormal velocity and WSS. Without stenosis, hemodynamics were significantly increased (Bonferroni corrected Mann-Whitney tests) in patients with bicuspid valves compared to patients with tricuspid valves. Incidence of elevated hemodynamics increased similarly for both cohorts (significant differences disappeared) with increasing stenosis severity.
Introduction
An increasing amount of evidence is emerging that hemodynamics are related to aortic dilation1–4. Recently, it was shown that regions with abnormally elevated wall shear stress (WSS) correspond with extracellular matrix dysregulation and elastic fiber degeneration in the ascending aorta (AAo) of patients with a bicuspid aortic valve (BAV), compared with regions of normal WSS5. Group-wise mapping of abnormally elevated aortic hemodynamics in patients with BAV or tricuspid aortic valves and aortic dilation (TAV+D) may help to clarify differences in aortopathy.Methods
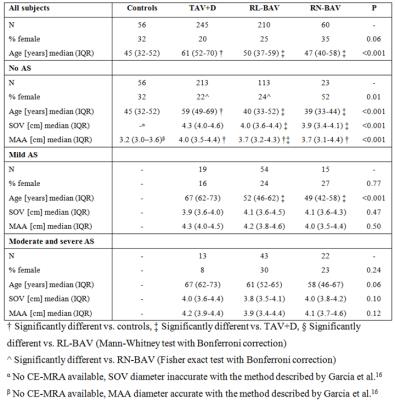
270 BAV and 245 TAV+D patients were retrospectively selected from a database. Dilation was defined as a sinus of Valsalva (SOV) or a mid-ascending aorta (MAA) diameter>4.0cm. BAV morphology was categorized into right-left (RL-BAV) and a right-noncoronary (RN-BAV) groups using b-SSFP images of the valve6. 56 healthy control subjects (TAV without dilation) were enrolled in the study. See table 1 for subject demographics.
4D flow MRI was performed with respiratory navigator gating and prospective ECG gating: spatial resolution=2.2–4.2×1.7–2.9×2.2-4.0mm3; temporal resolution=32.8–43.2ms; TE/TR/FA=2.2–2.8ms/4.1-5.4 ms/7-15°; VENC=150–400cm/s. All scans were performed on 1.5T and 3T MAGNETOM Avanto/Espree/Aera/Skyra systems (Siemens Healthcare, Erlangen, Germany).
Background phase and velocity aliasing corrections were performed. Phase contrast angiograms (PC-MRA) were created7. The aorta was segmented from the PC-MRA images in Mimics (Materialise, Leuven, Belgium). Peak systole was extracted by isolating the time frame with maximal spatially averaged absolute velocity in the segmentation. Peak velocity in the proximal ascending aorta (AAo) was assessed on a maximum intensity projection of the absolute velocity and used to categorize subjects in cohorts with no aortic stenosis (AS) (<2m/s), mild AS (≥2m/s and <3m/s) and moderate/severe AS (≥3m/s)8,9. WSS was calculated10.
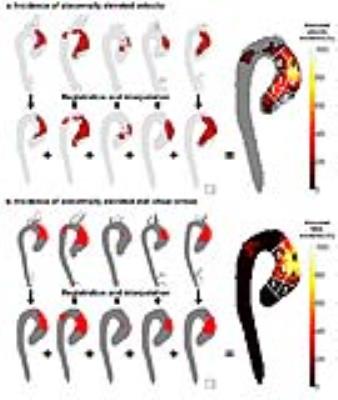
For age-matching of patients to healthy volunteers, five healthy velocity and WSS atlases were created: 19-30 years (n=11), 31-40 years (n=10), 41-50 years (n=19), 51-60 years (n=12) and 56-78 years (n=9). These were used to create ‘heat maps’ that represent patient-specific abnormally elevated velocity and WSS11. Subsequently, the heat maps were projected on cohort-specific ‘shared’ geometries12. By addition of the heat maps, a 3D map showing regional incidence of abnormally elevated velocity and WSS was created (figure 1). For quantification of the regional volume of elevated velocity and surface of elevated WSS, the ascending aorta (AAo) was subdivided in six groups: 1) the inner and 2) outer aortic root, 3) the proximal inner and 4) outer AAo, 5) the distal inner and 6) outer AAo (figure 1).
Results
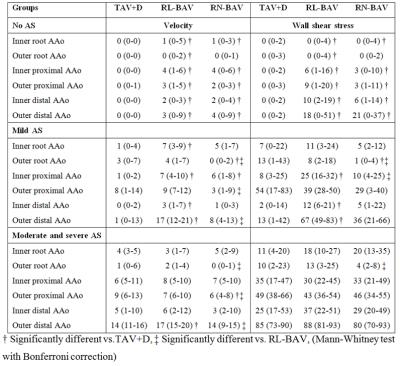
In figures 2 and 3 the maps and maximum incidence value for elevated velocity and WSS are shown. The velocity and WSS maps for RL-BAV (maximum 60%) and RN-BAV (maximum 57%) without AS show high incidence of elevated hemodynamics compared to TAV+D (maximum 23%). For each cohort, an increase of abnormal hemodynamics can be seen with increasing AS severity (figures 2, 3, table 2). With increasing AS severity the differences between the three cohorts disappeared. For mild AS, abnormal hemodynamics were found along the entire aorta for RL-BAV compared to localized abnormal hemodynamics for TAV+D (outer proximal AAo) and RN-BAV (outer distal AAo). The outer aortic root in RN-BAV showed a consistent pattern of low elevated hemodynamics for all AS categories.Discussion
With the novel methodology presented here, the grouping of patient-specific abnormal hemodynamics into regional incidence maps can provide a concise tool to investigate the pathological relationship between WSS and dilation. For example, increased WSS may correspond with known differences in aortopathy for RL-BAV (dilatation of root and the tubular AAo) and RN-BAV (dilatation of the distal AAo with sparing of the aortic root)13,14. The finding of low incidence of abnormal hemodynamics in the TAV+D group without AS aligns with the idea that WSS should be reduced in the presence of an enlarged aorta and no eccentric flow (when stroke volume is held constant).
The differences in abnormal hemodynamics can elucidate recent contradictions in literature, with Tsamis et al. stating that dilation occurs asymmetrically in BAV disease compared to symmetric dilation in TAV+D cohorts, and Girdauskas et al. stating that there was no difference in aortopathy between BAV and TAV+D patients with AS15,16. It is therefore important to stratify for AS. The addition of comparing abnormal velocity incidence maps helps to clarify underlying mechanisms of abnormal flow that lead to vessel wall remodeling in BAV disease.
Conclusion
Group-specific mapping of abnormal hemodynamics has the ability to visualize and quantify differences between groups of patients with different valve morphology and AS severity. The maps may help explain investigate the origins of aortopathy in these disparate groups.Acknowledgements
Grants: NIH R01 HL115828 and K25 HL119608References
1. Barker AJ, Markl M, Burk J, Lorenz R, Bock J, Bauer S, Schulz-Menger J, Knobelsdorff-Brenkenhoff F von. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012;5:457–466.
2. Mahadevia R, Barker AJ, Schnell S, Entezari P, Kansal P, Fedak PW, Malaisrie SC, McCarthy P, Collins J, Carr J, Markl M. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 2014;129:673–682.
3. Bissell MM, Hess AT, Biasiolli L, Glaze SJ, Loudon M, Pitcher A, Davis A, Prendergast B, Markl M, Barker AJ, Neubauer S, Myerson SG. Aortic dilation in bicuspid aortic valve disease: flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging 2013;6:499–507.
4. Meierhofer C, Schneider EP, Lyko C, Hutter A, Martinoff S, Markl M, Hager A, Hess J, Stern H, Fratz S. Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: a prospective study. Eur Hear J – Cardiovasc Imaging 2012;14:797–804.
5. Guzzardi DG, Barker AJ, Ooij P van, Malaisrie SC, Puthumana JJ, Belke DD, Mewhort HE, Svystonyuk DA, Kang S, Verma S, Collins J, Carr J, Bonow RO, Markl M, Thomas JD, McCarthy PM, Fedak PW. Valve-Related Hemodynamics Mediate Human Bicuspid Aortopathy: Insights From Wall Shear Stress Mapping. J Am Coll Cardiol 2015;66:892–900.
6. Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226–1233.
7. Bock J, Kreher W, Hennig J, Markl M. Optimized pre-processing of time-resolved 2D and 3D Phase Contrast MRI data. Proc Intl Soc Mag Reson Med 2007;15:3138.
8. Rose MJ, Jarvis K, Chowdhary V, Barker AJ, Allen BD, Robinson JD, Markl M, Rigsby CK, Schnell S. Efficient method for volumetric assessment of peak blood flow velocity using 4D flow MRI. J Magn Reson Imaging 2016.
9. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen W-K, Stevenson WG, Yancy CW. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg 2014;148:e1–e132.
10. Potters W V, Ooij P van, Marquering HA, VanBavel E, Nederveen AJ. Volumetric arterial wall shear stress calculation based on cine phase contrast MRI. J Magn Reson Imaging 2015;Feb; 41:505–516. 1
1. Ooij P van, Garcia J, Potters W V, Malaisrie SC, Collins JD, Carr JC, Markl M, Barker AJ. Age-related changes in aortic 3D blood flow velocities and wall shear stress: Implications for the identification of altered hemodynamics in patients with aortic valve disease. J Magn Reson Imaging 2016;43:1239–49.
12. Ooij P van, Potters W V, Nederveen AJ, Allen BD, Collins J, Carr J, Malaisrie SC, Markl M, Barker AJ. A Methodology to Detect Abnormal Relative Wall Shear Stress on the Full Surface of the Thoracic Aorta Using 4D Flow MRI. Magn Res Med 2015;Mar; 73:1216–1227.
13. Kang JW, Song HG, Yang DH, Baek S, Kim DH, Song JM, Kang DH, Lim TH, Song JK. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC Cardiovasc Imaging 2013;6:150–161.
14. Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014;370:1920–1929.
15. Tsamis A, Phillippi JA, Koch RG, Chan PG, Krawiec JT, D’Amore A, Watkins SC, Wagner WR, Vorp DA, Gleason TG. Extracellular matrix fiber microarchitecture is region-specific in bicuspid aortic valve-associated ascending aortopathy. J Thorac Cardiovasc Surg 2016;151:1718–1728.e5.
16. Girdauskas E, Rouman M, Disha K, Fey B, Dubslaff G, Theis B, Petersen I, Gutberlet M, Borger MA, Kuntze T. Functional Aortic Root Parameters and Expression of Aortopathy in Bicuspid Versus Tricuspid Aortic Valve Stenosis. J Am Coll Cardiol 2016;67:1786–96.
17. Garcia J, Barker AJ, Murphy I, Jarvis K, Schnell S, Collins JD, Carr JC, Malaisrie SC, Markl M. Four-dimensional flow magnetic resonance imaging-based characterization of aortic morphometry and haemodynamics: impact of age, aortic diameter, and valve morphology. Eur Hear J – Cardiovasc Imaging 2015:jev228.
Figures