0746
Improving B1 Field Efficiency and Reducing noise for In Vivo 31P MRSI with Ultrahigh Dielectric Constant Material1Center for Magnetic Resonance Research, Department of Radiology, University of Minnesota Medical School, Minneapolis, MN, United States, 2Center for NMR Research, Department of Radiology, The Pennsylvania State College of Medicine, Hershey, PA, United States, 33Department of Engineering Science and Mechanics, The Pennsylvania State College of Engineering, University Park, PA, United States
Synopsis
X-nuclei MRS for human application faces two challenges: high RF power requirement (thus, higher SAR) for achieving the same RF pulse flip angle due to a relatively lower gyromagnetic ratio, and limit of detection sensitivity (or SNR) even at high/ultrahigh field. In this work, we report that by incorporating ultra-high dielectric constant (uHDC) material into the RF head volume coil, huge RF transmit power reduction was observed in the regions near the uHDC pads for 31P MRSI at 7T. Concomitantly, the B1 efficiency for acquiring the spectra was increased about 100%. Strikingly, up to ~20% denoising effect was also observed with the uHDC material. Our results demonstrated that incorporating uHDC with RF coil can significantly boost SNR and reduce SAR in X-nuclei MRS applications; such improvements are beyond the gains obtainable at very high field strength magnet that has approached to its technologic limits.
Purpose
In vivo 31P MRSI provides an important neuroimaging tool for studying high-energy phosphate metabolisms, neuroenergetics and NAD redox state [1-2]. This study was to investigate the efficacy and utility of ultra-high dielectric constant (uHDC) technology for significantly improving SNR and reducing RF power requirement for in vivo 31P MRSI at ultrahigh field of 7T.Introduction
Increased RF power deposition or specific absorption rate (SAR) has become a major safety concern and technical hurdle at ultrahigh field despite its substantial improvement of the spatial/temporal/spectral resolution with increased signal-to-noise ratio (SNR). As one of the engineering solutions, incorporating high dielectric constant (HDC) material into the RF coil at various magnetic fields has provided substantial benefits for improving both the transmit efficiency (|B1+|) and receive sensitivity (|B1-|) [3-5]. Thus, our research aims were to 1) develop the ultrahigh HDC (uHDC) material using advanced material science for MR imaging application, and 2) to quantitatively investigate the B1 efficiency and sensitivity gain for 31P MRS application at 7T on both phantom and in vivo human brain validation. Here we present the first result of the B1 field improvements with the half-pipe uHDC material that was designed for the human occipital lobe.Methods
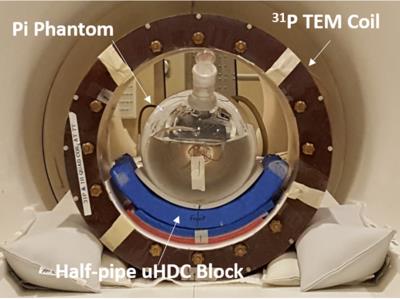
uHDC Material: First, given the operation frequency (120.3MHz for 31P at 7T) in conjunction with a 31P TEM head volume coil configuration, the ultrahigh effective permittivity constant (εeff = 1200) material was made lead zirconium titanate (TRS, State College, PA, USA). In particular, a half-pipe shape of the ceramic dielectric pad was successfully manufactured for targeting local region of the human occipital lobe. 31P MRS: All 31P MRS measurements were performed at 7.0T/90 cm bore human scanner (Siemens/Magnex) with 31P-1H double-tuned RF volume coil; 1H channel for anatomic imaging and B0 shimming, and 31P channel for acquiring 3D 31P chemical shift imaging (CSI). A phantom 31P MRS data were collected from a spherical container filled with inorganic phosphate (Pi: 100 mM) and gadolinium contrast agent for shortening the T1 value of Pi to ~300 ms. In vivo 31P MRS data were also collected on the brains of two healthy volunteers. The 3D 31P CSI data of the phantom were acquired with Fourier Series Window (FSW) technique [6] (TR= 1s, FA= 90°, phase encode= 9x9x7, bandwidth= 5 kHz, FOV= 20x20x18 cm3, and pulse width = 1 ms). In vivo 31P CSI data were acquired with slightly different parameters of TR= 1.5s with an Ernst flip angle. Finally, spatial maps of relative B1+ (i.e., inversely proportional to the RF transmit voltage for reaching a 90° RF flip angle) and B1- (proportional to the maximum signal at 90° flip angle) were calculated using fitting algorithm for determining the integrals of phosphorous creatine (PCr) signal for in vivo data and Pi signal for phantom data and their dependence on the variable RF pulse voltages. Noise was measured from the signal-free regions of the spectra.Results and Discussion
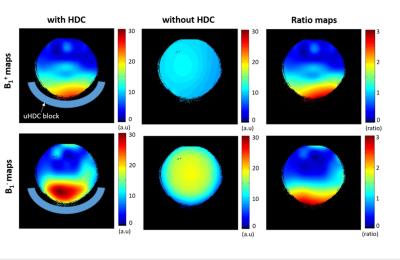
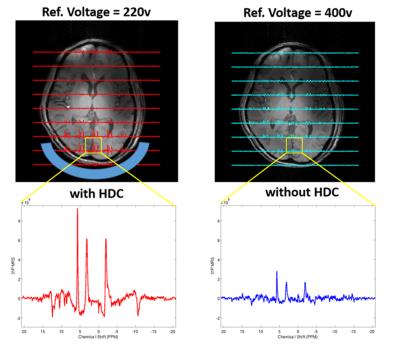
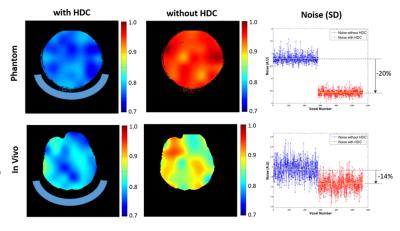
Figure 1 displays the experimental setup of the phantom and Fig. 2 shows both RF transmission field (B1+) and reception field (B1-) for acquiring 3D 31P CSI at 7T, which demonstrate large improvements with the uHDC pads for Pi phantom. Such a significant B1 improvements were also observed in human brain measurement showing an increased SNRs (more than 200%) and reduced RF power requirement (more than 200% voltage reduction) in the regions of interest near the uHDC pads as compared to that without pads (see Fig. 3). Strikingly, noise levels were also significantly reduced in both phantom (-20%) and in vivo case (-15%) with the use of the uHDC material (see Fig. 4). The amplitude of the denoising effect seems to depend on the configuration between the uHDC pad and RF volume coil as well as the loading condition. These results provide compelling evidence of the uHDC materials for improving B1 efficiency, consequentially leading to SNR enhancements and SAR reduction. Based on the field-dependent SNR of the PCr metabolite [7], a 200% SNR gain with the uHDC technique observed at 7T is equivalent to a similar performance and SNR expected at > 15T without additional concerns for SAR.Conclusion
Our results demonstrate that a novel design of uHDC materials significantly improved both |B1+| and |B1-| for in vivo 31P MRS. Therefore, utilizing uHDC materials could be an important and cost-effective engineering solution for overcoming high specific absorption rate (SAR) and significantly gaining SNR at ultrahigh fields, which will provide enormous benefits for in vivo human applications including brain research.Acknowledgements
NIH grants: R01 NS057560, NS070839, MH111447; R24 MH106049, S10RR026783, P41 EB015894, and P30 NS057091; and the AHC Faculty Research Development (FRD) grant from the University of Minnesota.References
[1] Du et al, PNAS 105:6409-14 (2008); [2] Zhu et al, PNAS 112:2876-81 (2015); [3] Qing et al, JMRI 38:435-440 (2013); [4] Lee et al., Proc. ISMRM; 23: 2382 (2016); [5] Rupprecht et al., Proc. ISMRM; 23: 403 (2014); [6] Garwood et al., JMR 75:244-261 (1987); [7] Lu et al, NMR Biomed 27:1135-41 (2014).Figures