0738
Targeted Delivery of Stem Cells to the Brain using Real Time Interventional MRI1Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Preclinical Parkinson’s Research Program, University of Wisconsin - Madison, Madison, WI, United States, 3Neuroscience Training Program, University of Wisconsin - Madison, Madison, WI, United States, 4Waisman Center, University of Wisconsin - Madison, Madison, WI, United States, 5Radiology, University of Wisconsin - Madison, Madison, WI, United States, 6Psychiatry, University of Wisconsin - Madison, Madison, WI, United States, 7Neuroscience, University of Wisconsin - Madison, Madison, WI, United States, 8Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
We present an intraoperative MRI protocol for stereotaxic surgery to precisely deliver induced pluripotent stem cells to targeted locations within the brain of a non-human primate model.
Previously, these surgeries were performed in stereotaxic operating rooms with no intraoperative imaging, or in a conventional MRI system without real-time guidance. Those environments complicate the goals of ensuring precise cannula tip placement before injection, and being able to perform the desired number of injections during the anesthesia window. Our platform enables surgeons to quickly achieve precise tip placement, and confirm via imaging that cells were deposited at the intended target.
PURPOSE:
The vast majority of in-vivo stem cell delivery procedures in complex pre-clinical and human studies are performed under stereotaxic guidance. MRI guidance and monitoring of stem cell delivery offers several advantages in monitoring the precise location of delivery and characterizing distribution, however the overall duration of the MR procedure imperils stem cell survival rates. We present an ensemble of rapid guidance and monitoring capabilities that promote the value of MR guidance while minimizing the interval cells reside in the dangerous period between bioreactor and injection. We demonstrate the value of the real-time intraoperative MRI (RT-IMRI) in the successful delivery of neuroprogenitor-induced pluripotent stem cells (iPSCs) to the putamen of 9 non-human primate (NHP) models of Parkinson’s disease in survival experiments spanning several months[1].METHODS:
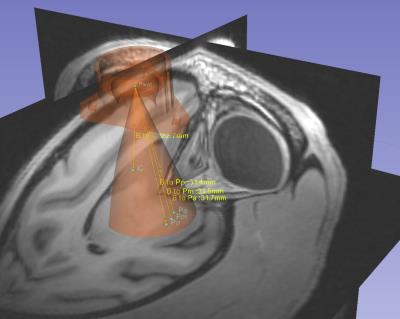
The real-time interventional MRI (RT-MRI) system consists of rapid cannula trajectory planning, device alignment, device insertion, and validation imaging after stem cell transplantation. Our system allows the operator to visualize the possible brain parenchyma trajectories, as shown in Figure 1, that are possible for any fixation point of a simple trajectory guide[2] (Navigus, Medtronic, Minneapolis, MN). The caudate and commissural putamen are both considered as potential iPSC targets, and the Navigus trajectory guide has a limited range of motion – a cone with apex angle of 36°. Thus, careful preoperative planning is required to determine a suitable mounting configuration for the guide, such that all targets will be reachable once the subject is in the MR bore. The trajectory guide is mounted overtop a burr hole roughly 10 mm in diameter and affixed to the skull with 3 small screws.
Once the desired trajectory has been selected, a computational approach is utilized to rapidly compute the centerline of the MR visible portion of the trajectory guide and align it with the desired trajectory. The computational approach, rather than imaging the entire alignment stem at high resolution[3], provides aiming feedback at 5 updates per second to the operator, enabling smooth intuitive motions and approximating the feel of stereotaxic OR tracking. All scanning was completed in a GE 3T 750 scanner (Waukesha, WI). A single channel 3-inch surface coil placed upon the skull around the trajectory guide provided signal reception.
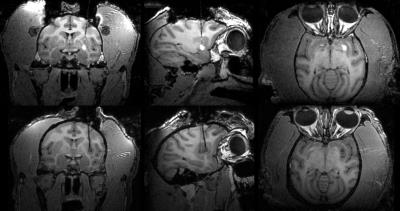
The large size of stem cells relative to other commonly infused agents limits prevents them from convecting, which limits the utility of an added Gd tracer for visualizing the cell distribution. Furthermore, the iPSC team wants to avoid introducing any Gd, as a precaution against metal toxicity in the vulnerable stem cells and surrounding tissue. Instead, we us an IR-prep spoiled gradient echo sequence which shows hypointense signal from the artificial cerebrospinal fluid (aCSF) buffering the cells. The difference between these contrast mechanisms is shown in Figure 2.
Minimizing the interval between transferring the iPSCs-derived neurons from the bioreactor, loading them into the cannula, and inserting the cannula from the surface of the brain to the desired target is crucial to maximize stem cell survival. Our RT-MRI system automatically determines and initiates scan planes collinear with the cannula trajectory to minimize this time.
RESULTS AND DISCUSSION:
Our protocol is able achieve a speed closer to that of the stereotaxic OR, while maintaining the key benefit of IMRI: the ability to acquire intraoperative images that enable good targeting precision. By moving faster than classic IMRI techniques, we are able to perform all the desired injections within the window of time that the subject may be under general anesthesia.
In each preoperative plan, we identified a location and orientation for the base was found that would allow all four potential targets to be reached from a single Navigus base. Each insertion and set of deposits was carried out within the window of time cells are capable of surviving within the cannula as determined by in vitro testing of the cannula/pump system [1] The negative contrast of the artificial CSF provides a good visual indicator of the injection location and extent (Figures 2, 3 and 4)
Quite often, the hypointense signal around a deposit will contain a small hyperintense center. The appearance varies from one deposit to the next (Figure 4) and we hypothesize that this may be due to variation in the clumping of the neurospheres.
CONCLUSIONS AND FUTURE WORK:
Additional in-vitro experiments may be warranted to investigate the observed variation in macroscale organization of the neurospheres after injection
It could be worthwhile to employ a bSSFP sequence that will yield high signal from CSF and could improve conspicuity of the cell deposit in early images, before the artificial CSF has diffused out into the brain tissue.
Acknowledgements
We acknowledge institutional support from GE Healthcare given to the Medical Physics and Radiology departments here. We thank HeartVista (Menlo Park, CA) for their technical support with the RTHawk software, and Graham Wright’s lab at the Sunnybrook Research Institute (Toronto, ON) for their work on the Vurtigo RT-IMRI visualization software.References
1. Vermilyea, S. C., Lu, J., Olsen, M., Guthrie, S., Tao, Y., Fekete, E. M., ... & Brodsky, E. (2016). Real-Time Intraoperative MRI Intracerebral Delivery of Induced Pluripotent Stem Cell-Derived Neurons. Cell Transplantation.
2. Hall, W. A., Liu, H., & Truwit, C. L. (2000). Navigus trajectory guide. Neurosurgery, 46(2), 502.
3. Truwit C and Liu H. Prospective stereotaxy: a novel method of trajectory alignment using real-time image guidance. JMRI. 2001;13(3):452–457.
4. Kalin, N. H., Fox, A. S., Kovner, R., Riedel, M. K., Fekete, E. M., Roseboom, P. H., ... & McFarlin, D. R. (2016). Overexpressing Corticotropin-Releasing Hormone in the Primate Amygdala Increases Anxious Temperament and Alters Its Neural Circuit. Biological psychiatry.
Figures