0737
MRI-guided robotic arm (MgRA) to target deep brain nuclei in vivo1Research Group of Translational Neuroimaging and Neural Control, High-Field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Graduate School of Neural Information Processing, Tuebingen, Germany
Synopsis
We develop a multiple degree-of-freedom robotic controlling system to target brain nuclei in the rat brain inside the high field (14.1T) MRI scanner. This MRI-compatible robot arm provides high targeting accuracy by using MRI images as a feedback to guide the brain intervention. Meanwhile, an MR-compatible camera-monitored insertion trajectory can be optimized through the controlling software in order to investigate the effectiveness, safety and feasibility of deep brain nuclei targeting for translational application.
Target audience
Scientists/radiologists who are interested in interventional surgeries with robotic
control, e.g. deep brain stimulation.Introduction
Recently, genetically encoded proteins make it possible to mediate and monitor the brain function from the molecular level to networks with cell specificity under multi-modal neuroimaging platform1. Specific brain cells can be activated optogenetically by delivering light pulse for photo-activation and the activity signal can be monitored inside the MRI-scanner with cell-specific calcium signal recording simultaneously with fMRI2-4. However, it remains challenging to accurately and precisely target the brain cells in specific functional nuclei of animal brains (only a few hundred micron size). The common way is to use the animal brain atlas to find the 3D coordinates based on a control point (zero point), which is defined by the bregma. The physical positon of the bregma point on the skull varies largely across animals with over a few hundred micron, which contributes to the key variation for most of the animal brain nuclei surgical procedures. There are clearly scientific needs for MRI-compatible, automatic robotic control, real-time guiding system to provide feasible targeting accuracy, high temporal and spatial resolution to guide the fiber intervention and monitor the collateral tissue damage during fiber insertion in order to investigate the effectiveness, safety and feasibility of deep brain nuclei targeting for translational application, e.g. deep brain stimulation, an implantable pump/needle for direct drug delivery, in multiple brain targeting tasks. The goal of this study is to develop a multiple degree-of-freedom robotic controlling system to target specific brain cells or deep brain nuclei in the rat brain inside the high field (14.1T) MRI scanner.Methods
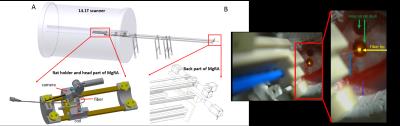
An MRI-compatible, automatic robotic control, real-time guiding multiple degree-of-freedom robotic arm5 has been developed for the 14.1T horizontal MRI-scanner with 12cm inner diameter gradient (Fig.1A). An MRI-compatible camera is implemented to obtain visual guidance of the fiber intervention. Fig.1B illustrates the camera-based signals for experiment setup in the iso-center of the horizontal bore magnet. The rat anesthetized with alpha chloralose is fixed by ear bars and tooth bar on the custom-designed MRI-compatible rat holder. A 24mm-diameter custom-designed transmit/receive surface coil is attached to the rat head while the fiber could be inserted into the brain through the hole of the coil driven by the robotic arm. The fiber is positioned above the brain craniotomy based on the visual guide. A 3D-MRI image is acquired to define the target and calculate the steps for movement by the operator. And the fiber is inserted into the brain step by step and real-time MRI images are acquired to monitor movement trajectory and identify the location of the fiber. MRI scans are performed using 2D rapid acquisition with relaxation enhancement (RARE) sequence: TR, 1200ms, TE, 7ms, 1.92cmX1.50cm FOV, 128X100 matrix, 0.15X0.15X0.8 mm3 spatial resolution. The step motor drives the head part to lower the fiber into the rat brain at each step and then started the next scan automatically until the fiber tip reaches the defined position. To avoid the electromagnetic interference, the robot controller and power supplies are placed in the scanner room at approximately 4.7 meters distance from the iso-center of the bore.Results
In this study, we demonstrated an MRI-compatible
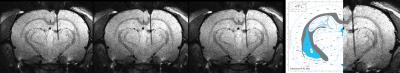
robotic arm for fiber placement in the 14.1T MRI scanner. Fig.2 shows the real-time
acquired MR images to target the lateral hypothalamus. The capability to place
the fiber with different depth is particularly useful to target multiple sites
along the insertion path. In addition,
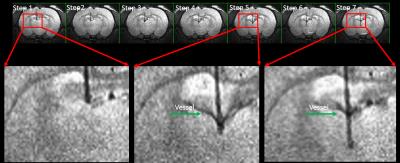
the collateral damage of the brain is visible during the insertion, as
illustrated in Fig. 3. When the fiber
tip touched the ventricle, the pushing force caused deformation of the
parenchyma tissue of the ventricle border, which could cause minor bleeding at
the choroid plexus. The precision of the
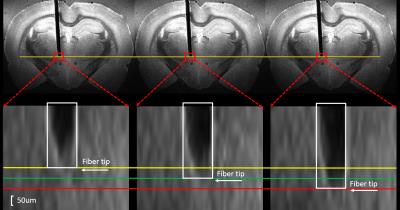
robotic arm along the dorsal-ventral axis is up to 50 µm. Fig.4 shows three
images with two steps to clarify the precision of the robotic arm in vitro.Conclusion
We developed a compact, MRI-compatible robotic
arm inside the MRI scanner to provide a flexible positioning system for fiber optic-mediated
multi-model fMRI methodologies in the rat brain. The MRI-guided robotic arm
provides visually monitored fiber insertion to reduce the position error
significantly and monitor damage of the brain during insertion in the rat
brain. The MgRA system can be translated to support human intervention surgical
procedures, e.g. deep brain stimulation, an implantable pump/needle for direct
drug delivery, in multiple brain targeting tasks.Acknowledgements
We thank Mr. Shanyi Yu for building up the first prototype of the robotic arm and Mr. Johannes Boldt for helping to improve the MgRA system. The financial support of the Max-Planck-Society and the China Scholarship Council (PhD fellowship to Yi Chen) are gratefully acknowledged.References
1. P. Rajasethupathy,E. Ferenczi, K. Deisseroth. Targeting Neural Circuits. Cell. 2016;165:524-534.
2. Yu X, He Y, Wang M, Merkle H, Dodd SJ, Silva AC and Koretsky AP. Sensory and optogenetically driven single-vessel fMRI. Nature Methods. 2016;13: 337-340.
3. J. H. Lee, et al. Bold claims for optogenetics. Nature.2010;465:788–792.
4. Kristina Schulz, et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nature Methods.2012;9:597-602.
5. Patent application number, EP 16195972.1 in Europe, "Positioning system for an imaging device".
Figures