0734
Detection of tumor hypoxic region using pH-activatable nanoparticles containing manganese contrast agent1Department of Molecular Imaging and Theranostics, National Institute for Radiological Sciences, National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan, 2Graduate School of System Informatics, Kobe University, Kobe, Japan, 3Institute of Innovative Research, Tokyo Institute of Technology, Japan, 4Innovation Center of Nanomedicine (iCONM), Kawasaki Institute of Industry Promotion, Kawasaki, Japan, 5State Key Laboratory of Biotherapy and Cancer Center, Sichuan University, People's Republic of China, 6Graduate School of Engineering, The University of Tokyo, Japan, 7Graduate School of Medicine, The University of Tokyo, Japan
Synopsis
Assessing the environment inside a tumor would aid the development of an effective therapeutic strategy. Our group has recently developed a pH-activatable nanoparticle containing MR contrast agents, which is called MnCaP micelle. For an in vivo tumor model, a specific and strong enhancement of MR signal in the tumor was obtained after the administration. The enhanced area was agreed with the high lactate region found with 1H-MRS and which corresponds to the hypoxic region inside tumor. We conclude that MnCaP micelle detects hypoxic regions in the tumor clearly and therefore it has potential to provide important information for tumor therapy.
Purpose
Evaluation of the tumor microenvironment, including vasculature, hypoxia
and inflammation, is one of most important factors in determining whether a
treatment strategy will succeeded or not [1, 2]. Much research into detecting
the hallmarks of the tumor microenvironment has been performed using imaging
techniques like PET and MRI [3-5]. For example, our group reported that nanoparticles
containing MR contrast agents can be utilized to detect the small tumors and
early-angiogenesis in the tumor region [6, 7]. Recently, we have also developed
a pH-activatable calcium phosphate (CaP) nanoparticle containing manganese as a
MR contrast agent, called MnCaP micelle to detect and evaluate differences in the
tumor microenvironment [8]. We present here studies evaluating in vivo MR signal changes in tumor using
MnCaP micelles to detect the low pH area related to the hypoxic region.Materials and Methods
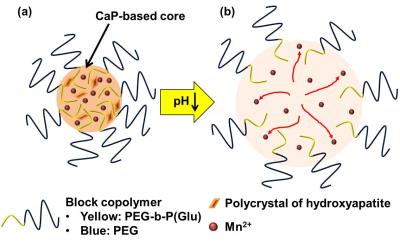
Nanoparticle: MnCaP micelles was comprised of Mn2+ encapsulated CaP nanoparticles made a poly(ethylene glycol) (PEG) shell and poly(ethylene glycol)-b-poly(glutamic acid) (PEG-b-P(Glu)) block copolymers (Fig. 1 (a)). The Mn ions were trapped in the CaP core with polycrystal structures of hydroxyapatite, and released from the CaP core at pH 7.0 (Fig. 1 (b)) [8]. The average diameter of the nanoparticle was controlled to be 60 nm.
MR experiments: All imaging experiments were performed at the National Institute for Radiological Sciences, Chiba, Japan. To detect and evaluate signal changes in the low-pH tumor region, MR experiments using female BALB/c nude mice transplanted with colon26 murine cancer cell (1.0 × 106 cells/50 μl) were performed. The mice were maintained in accordance with the guidelines of the institute, and all experiments were reviewed and approved by the institute's Committee for Care and Use of Laboratory Animals. The accumulation of MnCaP micelle in in vivo tumor was evaluated with T1-weighted spin-echo MRI using a 1.0 Tesla preclinical MR scanner (Icon, Bruker Biospin, Ettlingen, Germany). A 0.225 mmol/kg MnCaP micelle dose was administered to the tail vein of the tumor model mice. The same concentration of Gd-DTPA was administrated to a control group. The imaging parameters were as follows: TR/TE = 400/11.5 ms; FOV = 44.0 × 44.0 mm2; Matrix size = 256 × 256; Slice thickness = 1.0 mm; Number of slices = 10; Number of acquisition = 4. The chemical shift image of lactates was acquired using a 7.0 Tesla preclinical MR scanner (BioSpec, Bruker Biospin) with a cryogenic probe. The point-resolved spectroscopic (PRESS) sequence was used with the following parameters: TR/TE = 3000/20 ms; FOV = 14.1 × 14.1 mm2; slice thickness = 1.5 mm; acquired data number = 10 × 10 and matrix size = 16 × 16.
Results and Discussion
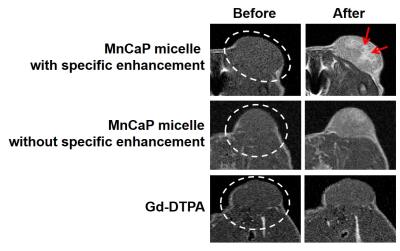
Figure 2 presents MR images before and
after the administration of the MnCaP micelle and Gd-DTPA. The tumor signal was
enhanced at 2 hours after MnCaP micelle administration. On the other hand, there
was almost no changes to the signal after the Gd-DTPA administration. Occasionally,
there is a specific signal enhanced area within the tumor as shown in the
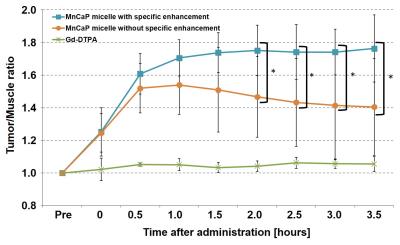
upper-right image of Figure 2. Figure 3 shows changes to the signal ratio after
MnCaP micelle and Gd-DTPA administration. The signal ratio after MnCaP micelle
administration was 1.5 times higher than the ratio before administration. For
the area with specific enhancement the signal ratio after MnCaP micelle
administration was higher than for areas without specific enhancement, and this
was maintained or increased from about 1 hour after the administration. It is likely
that the specific enhancement is associated with the release of Mn ion from the
CaP core and the subsequent binding with protein. Thus, the specific enhanced
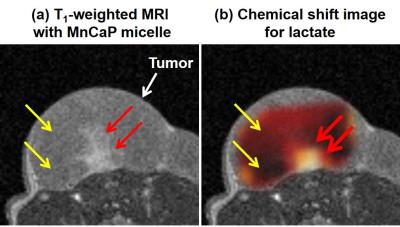
area is an indicator of the low-pH area. Figure 4 shows that the pattern of specific
signal enhancement using MnCaP micelle was similar to the concentration pattern
for lactate. The overproduction of lactate is related to an insufficient O2
supply and the acidified the interstitial pH. Therefore, this MnCaP
micelle can detect hypoxic regions within the tumor.
Conclusion
Our MnCaP micelle nanoparticle produced over 60% signal enhancements in the hypoxic tumor regions, and it therefore has the potential to provide the important information for tumor therapy.Acknowledgements
This research was financially supported by the Center of Innovation Program stream from the Japan Science and Technology Agency and the Funding Program for World-Leading Innovative R&D on Science and Technology from the Japan Society for the Promotion of Science. We thank Sayaka Shibata and Nobuhiro Nitta for assistance with MRI experiments, and also thank Jeff Kershaw for proofreading.References
[1] Hanahan D., et al: Cell, 144 (5): 646-674, 2011.
[2] Swartz M. A., et al: Cancer Res., 72(10): 2473–2480, 2012.
[3] Wehrl H. F., et al: J Nucl Med., 55 (5): 11S-18S, 2014.
[4] Heidari P., et al: J Nucl Med., 56 (8): 1246-1251, 2015.
[5] Zheng X., et al: Nat Commun., 5: 5834, 2015.
[6] Kokuryo D., et al: J Control Release, 169(3): 220-7, 2013.
[7] Kawamura W., et al: Sci. Technol. Adv. Mater, 16: 035004, 2015.
[8] Mi
P., et al: Nature Nanotechnology, 11 (8): 724–730, 2016.
Figures