0733
CEST MRI of PSMA expression using natural dextran-based contrast agents1F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 2The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University, Baltimore, MD, United States
Synopsis
It is desirable to have non-invasive imaging methods able not only to render the expression and distribution of receptors, enzymes and other targets relevant to
Purpose
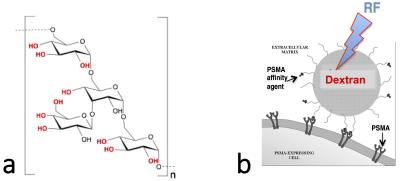
To develop a new receptor- or enzyme-based MR imaging approach using the clinically available agent dextran, which contains abundant hydroxyl protons enabling use for chemical-exchange-saturation-transfer (CEST) MRI (Fig. 1)1. Dextran is a highly sensitive, non-metallic, non-radioactive, and biodegradable diaCEST agent that can be functionalized easily with targeting ligands, enabling rapid clinical translation.Methods
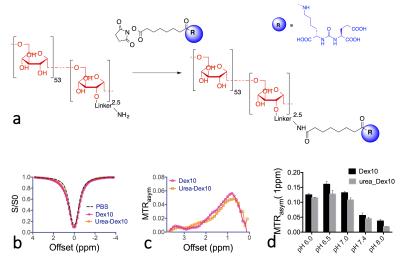
Dextran targeting the prostate-specific membrane antigen (PSMA) (Fig. 1b) was synthesized according to Fig. 2a. The binding affinity of urea-Dex10 to PSMA was measured using a standard, fluorescence-based assay2,3. For in vivo studies, both PSMA(+) PC3-PIP cells and PSMA(-) PC3-flu cells were inoculated in the opposite flanks of male SCID mice (n = 5). The in vitro CEST contrast of dextran was assessed using a vertical bore Bruker 11.7 T MRI scanner as described previously.4 In vivo MR studies were carried out on a Biospec11.7 T horizontal MRI scanner as described previously.5 CEST MR images were acquired before and within the first hour after the tail vein injection of dextran (100 mL, 375 mg/ml). For the dynamic study, CEST images were repetitively acquired at the offsets of ± 0.6, ± 0.8, ± 1.0, and ± 1.2 ppm using a modified fat-suppressed RARE sequence (CW saturation pulse, B1=1.8 µT and 3 seconds, TR/TE=5000/5 ms, RARE factor=10). To correct the B0 inhomogeneity, WASSR scans were acquired before and after the CEST acquisitions. The in vivo CEST contrast was quantified by averaging the MTRasym=(S-Δω – S+Δω)/S0 at 1.0 ppm. The change in CEST MRI was quantified by ∆MTRasym(t)= MTRasym (t)- MTRasym (t0).
Results
PSMA-targeted CEST MRI probes were synthesized by conjugating 10 kD dextran (Dex10, size ~ 4-6 nm) with a low-molecular-weight urea-based ligand (MW ~ 400). A high binding affinity to PSMA was confirmed in vitro, i.e. Ki = 2 nM (IC50 = 10 nM). The CEST signal of urea-Dex10 was found to be almost identical to that of the non-conjugated Dex10 (Fig. 2), attributed to only a small portion (i.e., 2.5 mmol/mole) of hydroxyl groups was functionalized in the conjugate. The CEST MRI detectability (1% MTRasym) was estimated to be approximately 20 µM.
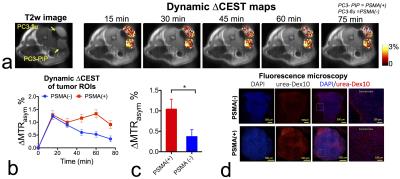
We then tested the urea-Dex10 in a well-validated prostate cancer xenograft model2. Our results showed that CEST MRI was able to detect the dynamic tumor uptake of urea-Dex10 (Figs. 3a, b). Initially (< 15 min), both tumors showed a substantial increase in CEST signal, i.e., 1.28 ± 0.16% and 1.23 ± 0.11% (∆MTRasym). Later (>15 min), CEST signal in PSMA(-) PC3-flu tumors decreased while that in PSMA(+) PC3-PIP tumors remained, indicating specific binding of urea-Dex10. At the final time point (60 min), the CEST enhancement (∆MTRasym) in PSMA(+) PC3-PIP tumors was approximately two times higher than that in PSMA(-) PC3-flu tumors, i.e., 1.33 ± 0.16% vs 0.53 ± 0.11%. A significant difference was found between the PSMA(+) and PSMA(-) tumors (P = 0.018, Student’s t-test, two-tailed and unpaired, n= 5, Fig. 3c), showing the ability to use urea-Dex10 to detect PSMA-expressing prostate tumors. Fluorescence microscopy (Fig. 3d) confirmed higher uptake of labeled urea-Dex10 in PSMA(+) PC3-PIP tumors than that in PSMA(-) PC3-flu tumors.
Discussion
We chose PSMA for proof-of-principle of targeted CEST MRI because of its importance as a target for imaging and therapy of prostate cancer. Although overall less sensitive than the corresponding methods from nuclear medicine, PSMA-targeted CEST MRI leverages the clinical ubiquity of MRI while serving as a platform for detection of other important receptors, enzymes and antigens present within malignant tissues. CEST MRI avoids the need for metallic species – and their attendant complications – to generate contrast.Conclusion
In summary, we have synthesized a new type of targeted MRI agent for PSMA-targeted imaging, and demonstrated its sensitive detection with CEST MRI in a relevant xenograft model. This prototype agent is comprised of diamagnetic dextran particles, which are currently being used clinically with a proven safety profile, and a small molecule urea-based PSMA-targeting ligand, which forms the basis of emerging clinical radiopharmaceuticals. The composition and sensitivity of this new agent portends a path to safe, rapid clinical translation.Acknowledgements
R21EB015609, R03EB021573, R01EB019934 and R01EB015032
References
(1) van Zijl, P. C., et al. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 4359-64.
(2) Banerjee, S. R., et al. Angew. Chem. Int. Ed. 2015, 54, 10778-10782.
(3) Chen, Y., et al. Clin. Cancer Res. 2011, 17, 7645-53.
(4) Liu, G., et al. Contrast Media Mol. Imaging 2010, 5, 162-70.
(5) Xu, X., et al. Magn. Reson. Med. 2015, 74, 1556-63.
(6) Chandran, S. S., et al. Cancer Biol. Ther. 2008, 7, 974-82.
Figures