0732
Chitin and dibutyrylchitin nanoparticles as imaging enabled vaccine nanocarriersBarbara Blanco Fernandez1, Yasser A Aldhamen2, Shatadru Chakravarty1, Andrea Amalfitano2, and Erik M Shapiro1
1Radiology, Michigan State University, East Lansing, MI, United States, 2Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI, United States
Synopsis
We describe a strategy for fabricating chitin and dibutyrylchitin –an organo-soluble chitin derivative- nanoparticles (NPs) encapsulating a model antigen (ovalbumin) or iron oxide nanocrystals. The use of MRI enabled the determination of the cell migration of vaccinated cells, allowing the determination of the immunization efficiency in early stages. To form DBC, a reversible acylation of chitin was performed and NPs were fabricated using an emulsification/evaporation method, then chitin was regenerated by alkaline saponification. NPs had vaccine adjuvant properties and allowed the track of immune cells to lymph nodes, proving their utility as vaccine adjuvants and imaging agents.
Introduction.
Chitin is a unique biodegradable and biocompatible polysaccharide with a pathogen-associated molecular pattern that activates TLR-2, regulating macrophage function and triggering the innate immune system in a vaccine adjuvant-like manner.1 Chitin nanoparticles (NPs) could serve as self-adjuvant vaccine nanocarriers and encapsulating both magnetic and antigen payloads would enable imaging to serially track the migration of vaccinated cells to the lymph nodes following either in vitro or in vivo vaccination.General Purpose.
The aim of this work was to prepare magnetic vaccine NPs using both chitin and an organo-soluble chitin derivative, dibutyrylchitin (DBC). To form DBC, a reversible acylation of chitin was first performed and NPs were fabricated using an emulsification/evaporation method. Afterwards, chitin was regenerated by alkaline saponification. DBC and chitin NPs were loaded with magnetic payloads or a model antigen (ovalbumin, OVA), and its performance as both imaging agent and vaccine adjuvants was tested.Methods.
DBC was synthetized from chitin as previously reported (Fig 1A).2 DBC NPs were prepared using two different approaches. Iron oxide nanocrystals (FeNC)-loaded NPs were prepared by oil-in-water emulsification with FeNC content ranging from 0-200% w/w respect to DBC. OVA-loaded DBC NPs were synthetized by water-oil-water emulsification with OVA content ranging from 0-100% w/w respect to DBC. To regenerate chitin, butyric groups were removed by alkaline saponification (0.5N KOH, 15 min, RT). The size and morphology of the NPs were studied with DLS, TEM and SEM. The loading content of FeNC and OVA was determined by TGA and BCA, respectively. The suitability of the system as vaccine carriers was tested by measuring OVA release and macrophage labeling. OVA release was performed in phosphate buffer pH 7.4 and the amount released was measured by BCA. Cellular internalization was studied by confocal microscopy (OVA-FITC loaded NPs) and TEM (FeNC-loaded NPs). For in vivo MRI, FeNC-loaded NPs were injected into mouse footpad (n=5) and the popliteal lymph nodes (PLN) were scanned at t=0, 24 and 48 hours. MRI parameters: 7.0T Bruker Biospec, Paravision 6.0, gradient echo, 3D flash T2*weighted, 60 micron isotropic resolution, TR: 40 ms, TE: 6.6 ms, FA: 15˚, scan time: 38 min. Following MRI, eosin/Prussian blue staining of PLN sections were done for confirming NPs presence. PLN cell suspensions sorted by magnetic cell sorting were stained with FITC-CD11C to confirm cell type. In order to check the immunopotenciator properties of NPs, empty and OVA-loaded NPs were incubated 48h with macrophages. Cellular activation was confirmed by measuring expression of CD80, CD86, MHC-II and CD40 by flow cytometry and the cytokines secreted (Bio-Plex Pro™).Results.
FTIR and 1H-NMR proved that the butyric groups were grafted onto both hydroxyl groups of the chitin to form DBC (Fig. 1B,C). DBC NPs were spheroidal with diameter 300-400 nm by DLS (Fig. 2A,C). Regeneration of chitin NPs was confirmed by FTIR with the loss of absorptions at 1740 cm-1 (Fig. 2D). Chitin NPs kept the morphology but were larger than DBC NPs (500-650 nm) (Fig. 2 A2,B2 and C2). NPs were able to encapsulate nearly 60% of FeNC with the higher ratios of FeNC tested (Fig. 2E) and 10% of OVA (Fig. 2F), and they were able to release OVA in a sustained rate (Fig. 2G). NPs were able to label macrophages after 1h incubation, and located in the cytosol (Fig. 3). Both chitin and DBC NPs were able to reach PLN following footpad injection, with DBC NPs being more efficient as shown by both MRI and histology (Fig. 4A and C). Flow cytometry on PLN cell suspensions showed 30-40% of magnetic labeled cells were CD11c+, a marker for macrophages and DCs (Fig. 4B). The immunopotenciator properties of chitin and DBC empty or OVA-loaded NPs was also assayed, indicating stimulation of CD40, CD80, CD86 and MHC-II production after 48h -even the blank NPs- and several cytokines related with the Th1 and Th2, especially in the case of OVA-loaded DBC NPs (Fig. 5).Discussion and conclusions.
A simple strategy for fabricating chitin and DBC NPs loaded with NC and antigens as OVA was described. NPs were able to reach the PLN and presented immunoactivity, establishing their utility as vaccine adjuvants. DBC NPs were better than chitin as vaccine adjuvants in vitro, simplifying the NPs vaccine synthesis. The use of MRI in this investigation, enabled by the incorporation of FeNCs in the NPs enabled the determination of the early time course of cell migration of vaccinated cells, allowing the determination of the immunization efficiency in early stages.Acknowledgements
NIH grants DP2 OD004362 and R21 EB017881, and a Michigan State University Foundation Strategic Partnership GrantReferences
(1) Da Silva CA, Hartl D, Liu W, et al. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation J. Immunol. 2008; 181: 4279-86. (2) Blanco-Fernandez B, Chakravarty S, Nkansah MK, Shapiro EM. Fabrication of magnetic and fluorescent chitin and dibutyrylchitin sub-micron particles by oil-in-water emulsification.Acta Biomater. 2016; 45: 276-285.Figures
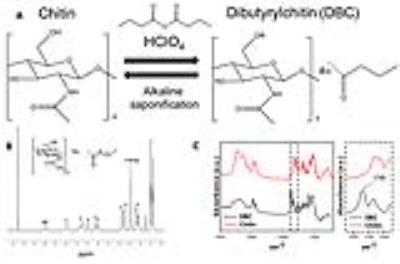
Figure 1. (A) Schematic of the DBC synthesis and the
alkaline saponification. (B) 1H-NMR of DBC in chloroform-d. (C)
FT-IR of chitin and DBC.

Figure 2. (A) SEM and (B, C) TEM of FeNC-loaded (A, B) and
OVA-loaded (C) DBC (1) and chitin (2) NPs, scale bar 200 nm. (D) FT-IR of DBC
and chitin NPs loaded with 50% FeNC. (E) FeNC loading content of the NPs. (F)
OVA loading content. (G) OVA release profile of the NPs in phosphate buffer pH
7.4 at 37 ˚C.
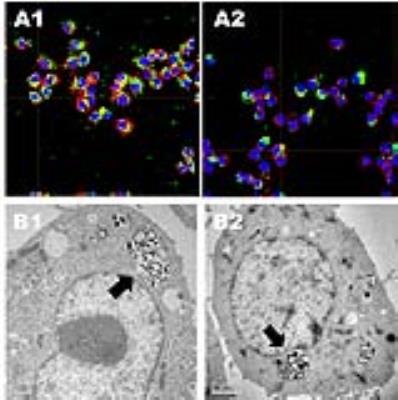
Figure 3. (A) Cellular labeling
of DBC (1) and chitin (2) NPs loaded with OVA-FITC by confocal microscopy after
1 h of incubation with Raw 264.7; in blue, nuclei stained with DAPI; in red,
cytoskeleton stained with phalloidin and in green, OVA-FITC-loaded NPs. (B) TEM
images of Raw 264.7 internalizing DBC (1) and chitin (2) NPs encapsulating
FeNC.
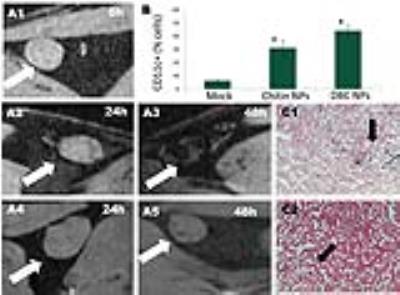
Figure 4. (A) Mice popliteal lymph node scanned with a Bruker 7T magnet before (1) and 24 h (A2, A4) and 48 h (A3, A4) of the administration of 50
µg of DBC (A2-3) or chitin (A4-5) NPs loaded with FeNC. (B) CD11c+ cells
encapsulating the DBC and chitin NPs encapsulating FeNC, cells were
magnetically sorted (values with an asterisk were statistical significant, P<0.001, one-way ANOVA, α=0.05, n=3). (C) Eosin/prussian blue staining ofa section of mice
popliteal lymph nodes 48h post-administration of DBC (C1) and chitin (C2) NPs
loaded with FeNC.
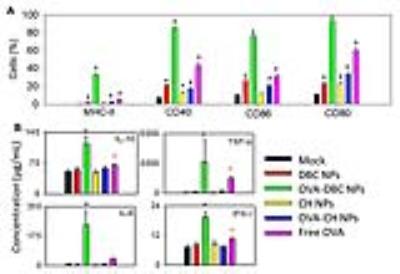
Figure 5. (A) CD86, CD80,
CD40 and MHC II expression in Raw 264.7 after 48h of incubation with only
medium, blank or OVA-loaded DBC and chitin NPs and free OVA (B) concentration
of IL-10, IL-6, TNF-α and IFN-γ by macrophages after 48 of incubation with
medium, blank or OVA-loaded DBC and chitin NPs and free OVA (same legend as in
(A)). Values with an asterisk were statistical significant (black asterisk P<0.001
and red asterisk P<0.05, one-way ANOVA, α=0.05, n=4).