0731
Molecular MR imaging of renal fibrogenesis in a mouse modelPhilip Alan Waghorn1, Iris Chen1, Nicholas Rotile1, Chloe Jones1, Diego Ferreira1, Lan Wei2, Bryan Fuchs2, and Peter Caravan1
1A.A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 2Division of Surgery, Massachusetts General Hospital, Boston, MA, United States
Synopsis
The fibrotic deposition and remodeling of extracellular matrix (ECM) proteins to form cross-linked collagen and elastin fibers is a characteristic feature of chronic kidney disease (CKD). Critical to fiber formation is the presence of allysine, which facilitates fibril cross-linking through condensation reactions with neighboring allysine and lysine residues on collagen and elastin. We have developed a novel Gd-based MR probe, GdOA, designed to target allysine for non-invasive molecular imaging of active fibrogenesis in kidney disease, allowing the onset of fibrogenesis to be detected and provide a quantitative measure of the efficacy of future anti-fibrotic targeted therapies.
Introduction
Chronic kidney disease (CKD) currently affects 12% of all adults in the US,1 with fibrosis and fibrogenesis identified as major amplifiers of CKD progression. If unchecked, fibrogenesis results in the deposition of fibrillar matrix proteins leading to enhanced kidney injury, accelerated nephron demise, diminished kidney volume and compromised perfusion, which can lead to kidney failure. Recent insights into the cellular and molecular mechanisms of fibrogenesis highlight the promise of new therapies to slow kidney disease progression. As such there is a need to be able to non-invasively image the extent of fibrogenesis in the kidney and to stage and monitor the response of disease to novel anti-fibrotic therapeutics. A key component of active fibrogenesis is allysine, a product of the lysyl oxidase oxidation of collagen. Allysine is fundamental for the crosslinking of collagen during active fibrosis and acts as a suitable biomarker for quantifying fibrogenesis.Purpose
The purpose of this work was to a) synthesize a novel Gd-based molecular MR probe, termed ‘GdOA’ for targeted imaging of allysine, b) validate the selectivity of GdOA binding and c) determine whether GdOA could quantify the extent of fibrogenesis in a mouse model of renal fibrosis.Methods
The Gd-probe, GdOA, was prepared in 7 steps from cyclen. To assess the allysine binding potential of GdOA, the probe was incubated with allysine-rich porcine aorta for 24 h at 37 °C, pH 7.4, and the extent of binding to allysine quantified by ICP analysis. To evaluate GdOA in an animal model of renal fibrosis, two cohorts of ten mice were studied: Group A) sham-treated, and Group B) a nephrotoxic nephritis (NTN) model. For the NTN group 129/SvEv mice were dosed at day 0 with 250 μg Sheep IgG and then with 125 μL sheep anti-rat GBM serum at day 5. Baseline images at 9.4T were measured 7 days post GBM serum injection. Animals were then injected with GdOA and imaged again at 24 h post GdOA injection. T1 maps of the kidneys were generated pre and post probe from a fast spin echo sequence with inversion recovery. Mean T1 values over the three major renal structures (cortex, medulla, and renal pelvis) were used to calculate changes in T1 due to binding of the probe. Animals were co-injected with EuDOTA as a non-binding control probe. Following MRI, kidneys were collected and assessed for hydroxyproline content (measure of total collagen), Gd content in tissue and for histology.Results
GdOA was synthesized from cyclen in high purity according to the scheme in Figure 1. Binding studies with allysine rich aorta (7.5 μmol allysine/g aorta) gave a Kd of 360 μM for GdOA. The relaxivity of GdOA was 4.25 mM-1s-1 in PBS and 8.10 mM-1s-1 when bound to allysine containing protein at 37 °C, 1.4T. The NTN model resulted in diffuse tubular injury, glomerulosclerosis, and mild fibrosis. In the NTN model of kidney fibrosis, GdOA resulted in a 6.9 fold increase in ΔR1 (probe relaxivity) in the cortex of the NTN group compared to control mice (P=0.048) (Figure 2c). Histograms of R1 distribution over the cortex show an R1 increase in NTN animals (Figure 3). No significant differences were observed for the medulla or renal pelvis, consistent with the distribution of fibrosis in this model. Imaging analyses were supported by ex vivo tissue analysis. Hydroxyproline content in tissue was 1.88 fold higher for NTN animals compared to control animals (P=0.0004) and Gd content in NTN kidney tissue showed a 4.64 fold increase compared to sham-treated mice (P=0.0032). (Figure 2a,b) The Gd:Eu ratio in kidney was elevated in the NTN model (1.41±0.09) compared to the control group (1.16±0.05) (P<0.001). The change in relaxivity, ΔR1, showed positive correlation with increasing hydroxyproline concentrations (r = 0.63).Discussion
The requirement of allysine for the crosslinking of extracellular matrix proteins during fibrogenesis make it is an appropriate marker of the extent of fibrotic disease in patients with CKD. GdOA is an oxyamine derivative of GdDOTA designed for targeted binding to allysine, with minimal off-target accumulation and rapid renal excretion. Binding studies with allysine rich aorta support selective binding of GdOA to allysine. Ex vivo tissue analysis suggests an increased fibrotic burden in the NTN animals, which correlates with an increase in GdOA uptake and MR signal enhancement in the NTN animals. The elevated Gd:Eu ratio in the NTN model indicates a selective retention of GdOA in fibrotic tissue as a result of selective binding to allysine.Conclusion
Non-invasive imaging with GdOA provides a means to detect and quantify the early stages of renal fibrosis.Acknowledgements
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (U01DK104302) and the Athinoula A. Martinos Center for Biomedical Imaging.References
1. J.S. Duffield. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Inv. 2014; 124:2299-2306Figures
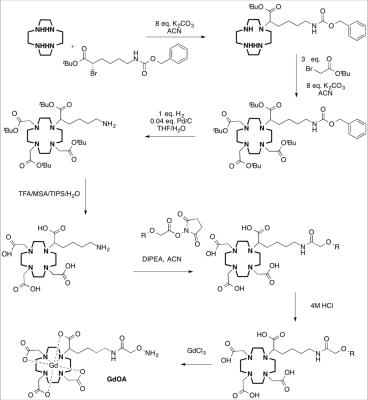
Figure 1: Synthesis of GdOA from Cyclen
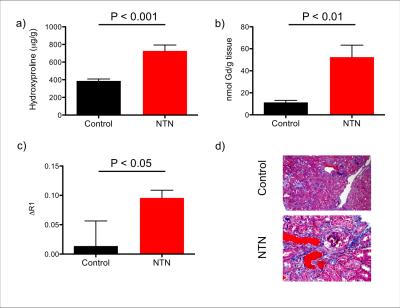
Figure 2: a) Hydroxyproline
levels, b) total Gd tissue content and c) change in renal cortex relaxation
rate are significantly increased in NTN animals compared to control mice, d)
Sample trichrome stains for control and NTN subjects showing increased tubular
injury and glomerular fibrosis with NTN model.
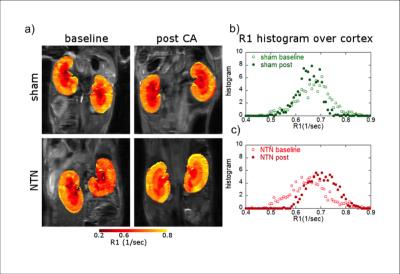
Figure 3: a) Coronal MR images with
overlaid kidney enhancement (shown in false color) for sham-treated and NTN
mice, pre- and 24h post-GdOA injection; b,c) Histograms of R1 distribution over
cortex in left kidney for sham-treated and NTN mice.