0730
19F-perfluorocarbon-labeled human peripheral blood mononuclear cells can be detected in vivo using clinical MRI parameters in a therapeutic cell setting1Microbiology and Immunology, Robarts Research Institute, London, ON, Canada, 2Medical Biophysics, Robarts Research Institute, London, ON, Canada, 3Medical Biophysics, Lawson Health Sciences Centre, London, ON, Canada, 4Cell Therapy Program, Krembil Research Institute, Toronto, ON, Canada, 5Urology, University of Western Ontario, London, ON, Canada
Synopsis
A major hurdle in advancing cell-based cancer vaccines is the inability to track where therapeutic cells migrate post-injection. We present the first study conducted in which primary Good Manufacturing Practice-grade 19Fluorine (19F) labeled all peripheral blood mononuclear cell lineages (PBMC) and 19F cellular MRI was used to track and quantify in vivo migration in a mouse model. Secondly, we present the highest sensitivity for 19F detection reported in the literature and the first time a 1.2cm deep injection of 19F-labeled PBMC using a small dual-tuned surface coil has been detected using a clinical scanner and MR protocol.
Purpose
With respect to antigen presenting cell-based (APC) cancer vaccines, the quantity of tumor antigen-specific APC reaching a secondary lymphoid organ post-injection is directly proportional to the magnitude of the ensuing anti-cancer immune response. We performed a pre-clinical validation study in order to progress towards a clinical trial. The main purpose, as a proof of principle study, was to determine if we could label human PBMC under GMP conditions with a 19F labeling agent and detect these cells in vivo at an immunotherapeutic-relevant depth using a clinical MR scanner.Methods
For manufacturing validation studies, human PBMC were obtained from 5 subjects and labeled with 19F (5mg/mL) overnight under GMP conditions. Phenotyping and viability assessment (using 7-AAD) took place both pre- and post-transport to Robarts Research Institute. Prior to in vivo imaging studies, NMR was performed to determine average 19F uptake per cell. Nude mice were then injected with 19F-PBMC and subjected to MR imaging.
Pre-clinical mouse MRI was conducted at 9.4T using a balanced steady state free precession (bSSFP) sequence with a resolution of 1x1x1mm3 for 19F scans and 200x200x200μm3 for 1H scans. 19F-human PBMC detection at both the subcutaneous flank injection site and draining lymph nodes was measured and quantified by using the in vivo 19F signal and comparing it to a 19F reference tube concentration.
Clinical in vivo MRI was performed on a 3T system using a dual-tuned surface coil (4.3cmx4.3cm) approved for human use. Intradermal mock injections of 19F-human PBMC ranging from 1x106 to 20x106 were performed in a ham shank as well as a subcutaneous injection of 4.5x106 cells 1.2 cm below the surface. A resolution of 0.5x0.5x1cm3 and scan time of 15min was used to acquire 19F-bSSFP images.
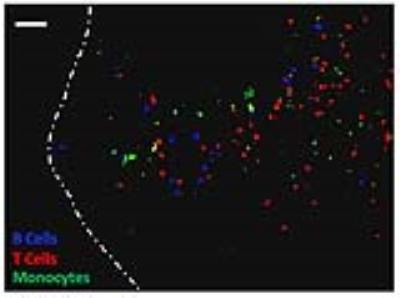
During this pre-clinical study, the question arose as to which main cell lineages (B cells, T cells and monocytes) within the heterogeneous PBMC formulation were producing in vivo signal in the lymph node. To address this important question, PBMC were negatively selected to purify out T cells, B cells and monocytes. Each lineage was then doubly labeled with 19F and a unique intracellular fluorophore. Prior to footpad injection of 3x106 PBMC in mice, lineages were recombined at physiologic proportions. Two days later, draining popliteal lymph nodes were removed, cryopreserved and sectioned for fluorescence microscopy to determine the degree of migration by each lineage.
Results/Discussion
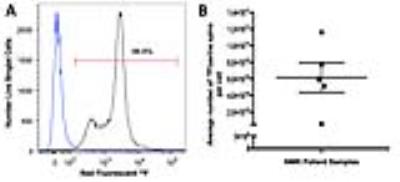
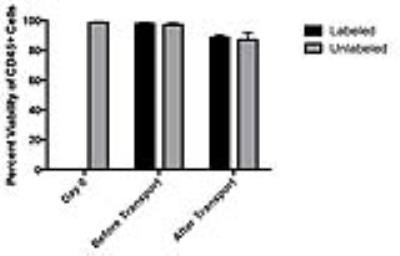
Our laboratory demonstrated near 100% 19F labeling of human PBMC and NMR revealed loading at 6.17x1010±1.80x1010 19F/cell (Figure 1A/B). GMP-labeling and transport of human PBMC had no significant effect on viability (Figure 2) and all sterility and quality assurance requirements were met. The functionality of APC within the PBMC mixture, as assessed by a mixed lymphocyte reaction, is unaffected by 19F-labeling. Together, this indicated that the current manufacturing process is suitable for an upcoming clinical trial. Human 19F-PBMC were detected and quantified in vivo in mice at both the subcutaneous flank injection site (Figure 3A/C) and draining lymph node (Figure 3B).
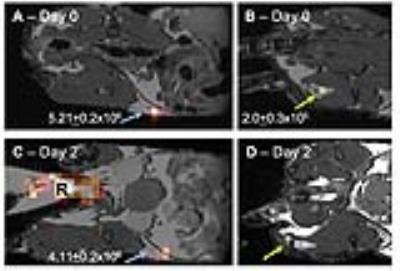
Clinical 3T imaging was first optimized using cell pellets in which as little as 4x1017 19F/spins were detected (data not shown). This to date is the highest sensitivity reported using clinical 3T parameters1, despite human PBMC labeling with low 19F spins per cell (6.17x1010). Next, MRI of a ham shank after surface intradermal and deeper subcutaneous (1.2cm, a depth similar to inguinal lymph nodes) injections ranging from 1-20x106 19F-PBMC permitted a minimum detection of 4.11x106 and 3.76x106 19F-labeled PBMC, respectively (Figure 4).
Immunofluorescent microscopy confirmed that the 19F lymph node signal was the result of originally injected PBMC and that all 3 of the main lineages within PBMC (B cells, T cells and monocytes) are present at physiologic proportions (Figure 5).
Conclusions
This proof of principle pre-clinical study provides a GMP-labeling procedure and experimental layout that yields near 100% 19F human PBMC labeling without affecting viability or compromising sterility and other quality assurance criteria that must be met for injection into humans. We outline a clinical 3T MR protocol approved for human use that is able to detect cell numbers in vivo an order of magnitude lower than what will be injected into prostate cancer patients upon clinical trial approval. Furthermore, we report the lowest sensitivity for 19F detection using a clinical set-up reported thus far in the literature and report the detection of a 19F signal deeper in vivo than what has ever been reported using a surface coil2. Further optimization is being performed while awaiting clinical trial approval to image 19F-labeled PBMC in humans.Acknowledgements
This pre-clinical study was supported by both a Movember Discovery Grant from Prostate Cancer Canada and the Smarter Imaging Program from the Ontario Institute for Cancer Research (OICR). JG is funded by the Sam Ciccolini Graduate Studentship from Prostate Cancer Canada. CF and JG are both members of the Western Molecular Imaging collaborative program and have received funding from the London Cancer Research and Technology Transfer (CaRTT) training program. CF has received funding from the Ontario Graduate Scholarship (OGS) Program.References
1) Ahrens ET, Helfer BM, O’Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med. 2014; 72(6):1696-701.
2) Rose LC, Kadayakkara DK, Wang G, Bar-Shir A, Helfer BM, O’Hanlon CF, Kraitchman DL, Rodriguez RL, Bulte JW. Fluorine-19 labeling of stromal vascular fraction cells for clinical imaging applications. Stem Cells Transl Med. 2015; 4(12):1472-81.
Figures