0728
Human Hyperpolarized C-13 MRI Using a Novel Echo-Planar Imaging (EPI) Approach1Radiology & Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Medicine, UCSF, San Francisco, CA, United States, 3Clinical Pharmacy, UCSF, San Francisco, CA, United States
Synopsis
In this pilot study, we developed and applied a specialized symmetric EPI approach for the study of hyperpolarized [1-13C]pyruvate metabolism in the human prostate. We show that this approach can acquire ghost-free hyperpolarized [1-13C]pyruvate and [1-13C]lactate images with high spatiotemporal resolution in a clinical setting.
Introduction
Clinical studies using hyperpolarized 13C substrates are currently underway at multiple research sites (1, 2). Phase 1 clinical studies (2) used slice-selective spectroscopy, single timepoint 3D echo-planar spectroscopic imaging (EPSI), or 2D EPSI to acquire data. While EPSI is robust to B0 inhomogeneities, it is slower than imaging-based approaches, requires offline processing, and is limited in spatial resolution because of the need for spectral encoding. Conversely, single-shot imaging based approaches, such as EPI (3), could provide significant benefits for clinical molecular imaging with hyperpolarized substrates. In addition to being more robust to motion and providing a higher temporal resolution, an imaging-based approach offers the ability to reconstruct 13C metabolite maps on the scanner in real time and can be readily incorporated into the existing clinical workflow. In this feasibility study, we developed and applied a specialized symmetric EPI approach for the study of hyperpolarized [1-13C]pyruvate metabolism in the prostate.Methods
Clinical imaging was performed on a 3T MR scanner using a 13C clamshell coil for RF excitation and a 1H/13C endorectal coil for reception. An 8M 13C urea phantom embedded in the endorectal coil was used to set the RF transmit power and center frequency.
Polarization: GMP [1-13C]pyruvate was prepared by a pharmacist and polarized using a 5T clinical polarizer (Spinlab, GE Healthcare) for two hours. The electron paramagnetic agent (EPA) was removed during dissolution, and pH, pyruvate and EPA concentrations, polarization and temperature were measured prior to injection. A 0.43mL/kg dose of 236mM pyruvate (polarization=36%) was injected at a rate of 5mL/s. The scan started 5s after the end of injection and 71s after the start of dissolution.
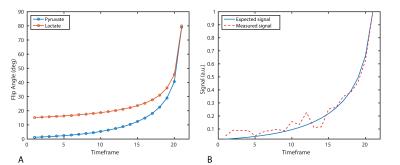
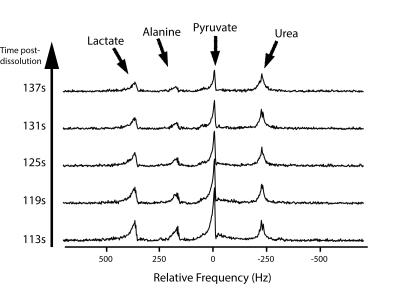
Hyperpolarized Imaging: 13C data were acquired with an 8x8mm in-plane resolution (12.8x12.8cm FOV, 16x16 matrix size), TR/TE = 62.5ms/15.4ms, echo-spacing = 0.62ms total acquisition time = 42s. Metabolites were sequentially excited with a singleband spectral-spatial RF pulse (150Hz FWHM, 600Hz stopband) using a variable flip angle scheme (Fig. 1). Sixteen 8mm slices were acquired, and the center frequency was alternated between pyruvate and lactate (Δf = 385Hz) for each volume, for an effective 2s temporal resolution. To correct for Nyquist ghosting, a reference scan was acquired on the 1H channel prior to 13C imaging (3). Following 13C imaging, non-localized spectra (TR = 3s, θ = 20°, 10 time-points) were acquired with a 500μs hard pulse to measure the metabolite frequencies.
Results & Discussion
The variable flip schedule employed here (Fig. 1) was designed to be robust in the presence of B1+ inhomogeneity. The 13C urea phantom was used for center frequency and RF calibration. Imaging the phantom with the pyruvate flip schedule confirmed the RF power calibration and phase correction coefficients prior to hyperpolarized imaging.
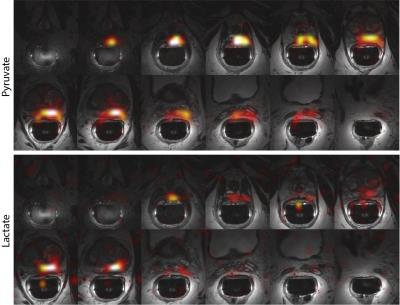
Area under the curve maps (Fig. 2) for pyruvate and lactate provide complete coverage from the base to the apex of the prostate with 8x8x8mm3 (0.5cm3) spatial resolution. Dynamic temporal resolution and volumetric coverage are crucial in translating this technology to the clinic, as a 2D slice approach could obfuscate or miss tumor location(s) while single time-point approaches are hampered by patient-to-patient variation in perfusion that will introduce quantification errors. No ghosting was visible in the phase-encode (L/R) dimension when using the reference scan acquired on the 1H channel, eliminating the need to directly acquire a reference scan from the hyperpolarized metabolites.
The ability to accurately set the transmit frequency is crucial to the success of imaging-based approaches, as off-resonance will result in a bulk shift in the phase-encode (blip) dimension for EPI studies. While no shift was observed here (Fig. 3), poor B0 homogeneity or larger receive frequency errors can be corrected for by phase-modulating the data in k-space or by reversing the blip polarity every other timeframe (4).
In this study, the total readout time was less than 10ms. While the peak SNR for pyruvate and lactate was 45 and 14, the SNR could be readily improved by increasing the total readout duration. However, this will place more of a burden on shimming and frequency calibration since the increase in echo-spacing would make the acquisition more susceptible to geometric distortion and bulk shifts in the blip dimension.
Conclusion
To our knowledge, these results represent the first hyperpolarized 13C data acquired in the human prostate with an imaging-based sequence. In this feasibility study, we show that symmetric EPI can be used to acquire ghost-free hyperpolarized metabolite maps with high spatiotemporal resolution in a clinical setting. As an alternative to spectroscopic imaging, this metabolite-specific EPI acquisition is more robust to motion and can be easily incorporated into existing diagnostic workflows.Acknowledgements
This work was supported by NIH grants R01EB017449, R01CA183071 and P41EB013598.References
1.) Cunningham, C.H., et al., Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circulation Research, 2016.
2.) Nelson, S.J., et al., Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Science Translational Medicine, 2013. 5(198): p. 198ra108.
3.) Gordon, J.W., D.B. Vigneron, and P.E.Z. Larson, Development of a Symmetric EPI Framework for Clinical Translation of Rapid Dynamic Hyperpolarized 13C Imaging. Magnetic Resonance in Medicine, 2016: p. DOI: 10.1002/MRM26123.
4.) Cunningham, C.H., et al., Frequency correction method for improved spatial correlation of hyperpolarized 13C metabolites and anatomy. NMR in Biomedicine, 2014. 27(2): p. 212-218.
Figures