0727
Utilizing hyperpolarized MRI in prostate cancer to assess metabolic dynamics and histopathologic grade1Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Molecular Pharmacology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Radiochemistry & Imaging Probes (RMIP) Core, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 4Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 5GE Healthcare, Toronto, ON, 6Berkshire Magnetics, Berkeley, CA
Synopsis
A hallmark of prostate cancer is the reprogramming of prostate cancer metabolism which has been exploited for both 31P and 1H MRSI. In recent work, we and others have shown that hyperpolarized substrates can be used in living systems to measure changes in metabolic dynamics. Many studies have focused on the use of HP pyruvate in preclinical models, though the characterization of prostate cancer in man using HP MRI has been limited. In this work, we demonstrate the use of HP pyruvate MRI in prostate cancer patients. We assess the metabolic dynamics to the prostate of pyruvate as well as its conversion to lactate. Moreover we extend this analysis to the comparison of HP lactate to prostate cancer grade showing that not only does this approach have the potential to measure differences in grade it also can provide reproducible metabolic signatures across patients.
Purpose
It has been shown that prostate metabolism is reprogrammed with oncogenesis resulting in increased glycolytic metabolism to lactate1. Hyperpolarized magnetic resonance imaging (HP MRI) utilizing dissolution dynamic nuclear polarization with pyruvate has been used in preclinical animal models2 and in vitro tissue culture models to correlate increased metabolism with tumor grade3. Utilizing hyperpolarized MRI in prostate cancer to assess metabolic dynamics and histopathologic grade. Recent work has shown that HP pyruvate is safe for use in patients4. It is unclear whether measurements of HP lactate can be used both reproducibly in patients and to grade prostate cancer. In this study, we aim to develop and assess methods to measure the delivery dynamics of HP pyruvate to the prostate as well as its conversion to lactate. In double patient injections, we explore the reproducibility of pyruvate and lactate dynamics. Moreover, with corresponding histopathology, we aim to characterize the ability of HP MRI to assess histopathologic grade across patients and in paired measurements.Methods
12 men (60.5±7.7yr) with biopsy proven prostate lesions >1cm (PSA 10.3±6.9ng/mL) were scanned using an IRB-approved protocol (IRB#14-205), of which all went on for total prostatectomy. Five men were re-injected and scanned a second time within 1hr to assess reproducibility. For the HP scans, we injected 0.43mL/kg of [1-13C]pyruvate intravenously (5mL/s). In accordance with our IND Acknowledgement (IND#125947), the patient doses were prepped in a clean room facility and subjected to a Quality Control (QC) check to ensure patient safety. Data were acquired at 3T with a dual-tuned 13C/1H endorectal-probe. T2-weighted images were acquired for anatomic reference. HP data were acquired using a 2D dynamic EPSI sequence5. The spatial and temporal resolution were 1x1x1.5cm3 and 5.7s. The data were reconstructed with a spatial resolution of 0.5x0.5x1.5cm3. To correct for delivery of pyruvate to the prostate we identified an arterial input function in the field of view (internal pudendal artery) and normalized using the time-to-max. The area under the pyruvate and lactate peaks were used to assess metabolic dynamics of the tumors and normal prostate. Time-to-max of pyruvate and lactate were compared between patients using a T-test. Subjects with two injections were assessed using a paired T-test. The ratio of maximum lactate to total carbon signal (maxLac/tCarb) was compared to histopathology results. This ratio was compared to histology grade using an ANOVA test; the ratios in subjects with two injections were compared using a paired T-test.Results and Discussion
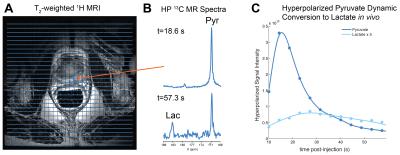
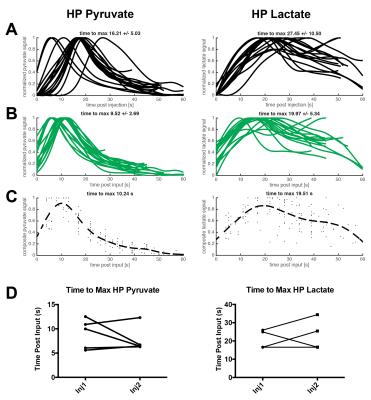
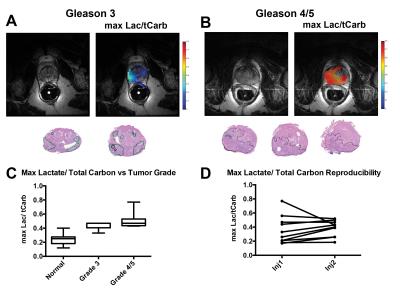
Utilizing the 2D dynamic EPSI, we were able to acquire spatially resolved data from N=17 injections and assessed both pyruvate delivery and conversion to lactate (Fig.1). Taking all injections into account, we measured an average time-to-max for pyruvate of 16.21±5.03s and lactate 27.45±10.50s (Fig.2A), which was dramatically reduced when accounting for the arterial input function 9.52±2.69s and 19.97±5.34s for pyruvate and lactate (Fig.2B). This allowed for calculation of an average delivery curve, which can be used for future 3D imaging planning (Fig.2C). When assessing reproducibility of HP pyruvate delivery and lactate generation in five subjects, no significant difference was observed for either metabolite (p=0.39 and 0.6,Fig.2D). We compared the spatial distribution of HP lactate to histopatholgy of the prostatectomy samples. The sections were matched to the imaging slice for each case and regions of dominant Gleason 3 (green) and Gleason 4/5 (black) were highlighted by a pathologist (Fig.3AB). Comparing normal prostate to tumor regions, a significant increase in the Lactate/Total Carbon ratio was measured (P<0.001,Fig.3C). In addition, an increase in the ratio was observed when comparing Gleason 3 and 4/5 lesions, though more patient samples will be needed to approach significance. When comparing regions of normal (n=5) and tumor (n=5) in sequential injections, we observed no significant difference in the Lactate/Total Carbon ratio (p=0.2,Fig 3D), demonstrating that HP MRI has the potential to make reproducible measurements of metabolic changes even on this short acquisition time scale.Conclusions
We have demonstrated that the delivery of pyruvate and its conversion to lactate are observable in human prostate. The time-to-max-pyruvate is reproducible when corrected for the arterial input function with no significant difference observed in repeat injections of the same patient. The HP Lactate signal is spatially correlated with tumor as delineated on histopathology and the Lactate/Total Carbon ratio can potentially be used to delineate regions of prostate cancer in patients. Future studies will include more patients and 3D acquisitions to cover the entire prostate. The reproducibility of these measurements and the ability to normalize for HP pyruvate delivery supports the great potential for using HP pyruvate MRI in the characterization of prostate tumor biology in humans.Acknowledgements
We would like to acknowledge support from the National institutes of Health (NIH R00 EB014328, S10 OD016422, and R01 CA195476), The Center for Experimental Therapeutics at MSKCC, Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and The Sir Peter Michael Foundation.
References
1Costello Franklin Costello LA, Franklin RB. The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology. 2000 Nov 16;59(4):269-82.
2Albers MJ, Bok R, Chen AP, Cunningham CH, Zierhut ML, Zhang VY, Kohler SJ, Tropp J, Hurd RE, Yen YF, Nelson SJ. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Research 2008; 68(20):8607-15.
3Keshari KR, Sriram R, Van Criekinge M, Wilson DM, Wang ZJ, Vigneron DB, Peehl DM, Kurhanewicz J. Metabolic reprogramming and validation of hyperpolarized 13C lactate as a prostate cancer biomarker using a human prostate tissue slice culture bioreactor. The Prostate. 2013 Aug 1;73(11):1171-81.
4Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C] pyruvate. Science Translational Medicine 2013; 5(198):198ra108.
5Chen AP, Cunningham CH, Ozturk-Isik E, Xu D, Hurd RE, Kelley DA, Pauly JM, Kurhanewicz J, Nelson SJ, Vigneron DB. High-speed 3T MR spectroscopic imaging of prostate with flyback echo-planar encoding. Journal of Magnetic Resonance Imaging 2007; 25(6):1288-92.
Figures