0726
Demonstrating the Randle Cycle In Vivo: Assessment of Physiological Alterations in Human Cardiac Metabolism Using Hyperpolarised 13C MR Spectroscopy1Department of Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom, 2Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom, 3Physical Sciences, Sunnybrook Research Institute, Toronto, Canada, 4Department of Physics, University of Oxford, Oxford, United Kingdom
Synopsis
The recent introduction of dissolution Dynamic Nuclear Polarization (DNP) has opened up a new window on in vivo metabolism and in this work we present the first demonstration that dissolution-DNP can observe physiological modulation of metabolism in the healthy human heart. The transition from the fasted to the fed state is shown to lead to an increase in flux through the key regulatory enzyme, pyruvate dehydrogenase, due to a metabolic switch away from fatty acid oxidation towards glucose oxidation. Such studies will provide the basis for future clinical studies exploring the metabolic alterations that occur in the diseased heart.
Introduction
The Randle (Glucose Fatty-Acid) cycle was first proposed by Sir Philip Randle in 1963 to describe the metabolic competition between fatty acids and glucose within cells1. One of the key sites of the regulation of this metabolic balance is the enzyme pyruvate dehydrogenase (PDH), which catalyses the oxidative decarboxylation of pyruvate into acetyl-CoA and CO2. The recent introduction of dissolution Dynamic Nuclear Polarization (DNP) has opened up a new window on in vivo metabolism2 and has been widely used to demonstrate physiological and pathological changes in pyruvate metabolism in the rodent heart3-6. In this work, we present the first demonstration that dissolution-DNP can observe the physiological modulation of metabolism (through assessment of the transition from the fed to the fasted state) in the healthy human heart, thereby demonstrating the Randle cycle in the human heart in vivo.Method
All scanning was undertaken on a 3T Tim-Trio MR System (Siemens, Germany) and was approved as a physiological study by both local and national research ethics committees. Three healthy male participants were recruited (Age=50±3yr, Body Weight 69±8kg) and provided written informed consent to participate in the study. Participants were initially scanned in the fasted state following on overnight fast of >8hr. They subsequently received an oral glucose load of 70g (450ml Lucozade, Coleford, UK) and were rescanned one hour later. Scanning consisted of 1H CINE imaging for functional assessment, 1H localised spectroscopy for assessment of myocardial triglyceride levels, 31P MR spectroscopy for assessment of cardiac energetics and 13C MR spectroscopy for assessment of PDH flux. Hyperpolarized [1-13C]pyruvate was generated in a SpinLab hyperpolarizer system (GE Healthcare, USA) and, following appropriate quality control measurements, was transferred to a Medrad syringe ready for patient injection. A bolus of 0.4ml/kg was injected at an infusion rate of 5ml/s, followed by a 20ml saline flush. Immediately before the injection, a cardiac-gated, slice selective, 13C pulse-acquire sequence was initiated to acquire in vivo cardiac spectra for the following 4mins (TR=500ms, FA=10°, BW=5kHz, 2048 complex points, slice thickness=10cm). Spectra were localised to the heart through the use of slice selection and a two-channel transmit, eight-channel receive surface coil placed directly over the heart. Multi-coil data were reconstructed by WSVD7, prior to thermal baseline subtraction and quantification by AMARES8. Maximum metabolite levels were then normalised to maximum pyruvate levels.Results
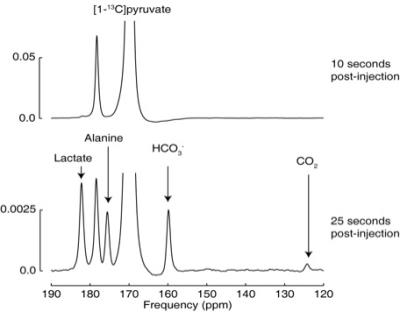
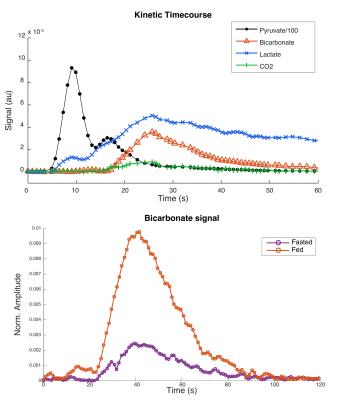
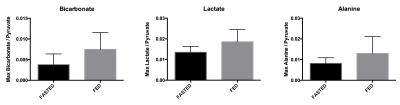
All injections were well tolerated with no adverse effects. Hyperpolarized pyruvate (250±10mM, >40mL) was obtained with polarization levels of 40±4% at 33±1°C and pH 7.4±0.5, with a low residual paramagnetic radical concentration of 0.6±0.4μM. Figure 1 shows example spectra acquired at 10s and 25s after the start of the injection into a fasted control participant, showing pyruvate arrival (10s) and metabolic conversion into lactate, alanine, bicarbonate and carbon dioxide (25s). Figure 2 shows the kinetics of hyperpolarized pyruvate and its metabolic products. As can be seen in Figure 3, the transition from the fasted to the fed metabolic state induced by the 70g oral glucose load led to a 97% increase in the metabolic conversion of pyruvate into bicarbonate (Fasted=0.0038±0.0018, Fed=0.0075±0.0029), indicative of an increase in flux through the key regulatory enzyme, pyruvate dehydrogenase. No significant changes in the conversion of pyruvate to either lactate (Fasted=0.014±0.002, Fed=0.019±0.004) or alanine (Fasted=0.008±0.002, Fed=0.013±0.006) were observed.Discussion and Conclusion
This study has demonstrated the feasibility of undertaking metabolic studies with hyperpolarized pyruvate in the human heart. As proposed by the Randle cycle, the transition from the fasted to the fed state has led to an increase in the flux of pyruvate through PDH due to a metabolic switch away from fatty acid oxidation towards glucose oxidation. Such studies will provide the basis for future clinical studies exploring the metabolic alterations that occur in the diseased heart and the interactions between metabolism and function that may provide new insight into the development of novel therapeutics.Acknowledgements
The authors would like to acknowledge financial support from the British Heart Foundation (Fellowships FS/10/002/28078 & FS/11/50/29038, Programme Grant RG/11/9/28921) and the OXFORD-BHF Centre for Research Excellence. We also acknowledge financial support from the National Institute for Health Research Oxford Biomedical Research Centre program and EPSRC Doctoral Training Centre Grant & Doctoral Prize Fellowships (refs. EP/J013250/1 and EP/M508111/1). The authors would also like to acknowledge support from the University of Cambridge in the preparation of the sterile fluid pathways used in this study.References
1. Hales, C.N. and P.J. Randle, Effects of low-carbohydrate diet and diabetes mellitus on plasma concentrations of glucose, non-esterified fatty acid, and insulin during oral glucose-tolerance tests. Lancet, 1963. 1(7285): p. 790-4.
2. Ardenkjaer-Larsen, J.H., et al., Increase of signal-to-noise of more than 10,000 times in liquid state NMR. Discov Med, 2003. 3(19): p. 37-9.
3. Schroeder, M.A., et al., In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proc Natl Acad Sci U S A, 2008. 105(33): p. 12051-6.
4. Atherton, H.J., et al., Role of pyruvate dehydrogenase inhibition in the development of hypertrophy in the hyperthyroid rat heart: a combined magnetic resonance imaging and hyperpolarized magnetic resonance spectroscopy study. Circulation, 2011. 123(22): p. 2552-61.
5. Dodd, M.S., et al., Impaired in vivo mitochondrial Krebs cycle activity after myocardial infarction assessed using hyperpolarized magnetic resonance spectroscopy. Circ Cardiovasc Imaging, 2014. 7(6): p. 895-904.
6. Le Page, L.M., et al., Increasing Pyruvate Dehydrogenase Flux as a Treatment for Diabetic Cardiomyopathy: A Combined 13C Hyperpolarized Magnetic Resonance and Echocardiography Study. Diabetes, 2015. 64(8): p. 2735-43.
7. Rodgers, C.T. and M.D. Robson, Receive array magnetic resonance spectroscopy: Whitened singular value decomposition (WSVD) gives optimal Bayesian solution. Magn Reson Med, 2010. 63(4): p. 881-91.
8. Naressi, A., et al., Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med, 2001. 31(4): p. 269-86.
Figures