0721
Improved tractography-based segmentation of the human thalamus1Translational Imaging Group, UCL, London, United Kingdom, 2MRI Unit, Epilepsy Society, Chalfont St Peter, United Kingdom, 3Icometrix, Leuven, Belgium, 4Dementia Research Centre, UCL, London, United Kingdom
Synopsis
Accurate segmentation of the thalamus and its nuclei is a prerequisite for studying anatomical connectivity and its correlation to neurological diseases. The probabilistic tractography pipeline in FSL is commonly used for thalamus connectivity-based parcellation. However, dMRI data analysis and tractography are done in a mix of standard and subject spaces which can bias anatomical connectivity findings. Here, we presented a framework that improves thalamus parcellation by performing DW data processing and probabilistic tractography in the subject’s native space, as well by generating population- connectivity priors. Higher segmentation accuracy was achieved with it when compared to FSL’s available pipeline.
Introduction
The thalamus is the main gateway to the cerebral cortex for the sensory, motor and limbic systems. It is
composed of distinct nuclei that differ in terms of the existing neural pathways.
FSL provides a probabilistic tractography pipeline to parcellate the thalamus [1,2] which has been widely
used to study anatomical connectivity. However, it is limited by its reliance on registration to propagate
seed and target regions of interest (ROIs) defined in a standard template space. The more different a
subject's morphology is from the template, the more potential there is for registration errors that will
affect the final tractography measurements leading to spurious connectivity effects.
We propose a subject-specific and probabilistic framework to avoid problematic registrations to standard
spaces and the associated potential for bias. Introduction
Methods
Methods
Data processing
Diffusion-weighted imaging (DW) was performed on 15 healthy individuals. Data was acquired twice along 64 non-collinear isotropic directions (b-value=1000 s/mm2, 2.5 mm3 vox) and four additional b-0 volumes. Volumetric T1-weighted MPRAGE images were also acquired (1.1 mm3 vox).
DW data was corrected for motion, eddy-current and susceptibility artefacts using NiftTK [3], while T1- weighted images underwent bias correction and brain parcellation into 143 regions [4]. T1- weighted data, thalamus and cortical segmentations were propagated to the DW space through intra-subject registration.
Tractography
Multi-fibre orientations were inferred through constrained spherical deconvolution (CSD) [6]. Then, probabilistic tracking was done from the thalamus (seed) to each cortical region (target) using iFOD2 [7] (lmax=8 and total_tracts = 5000). Probability maps were derived for each target region’s connection likelihood.
Proposed segmentation framework
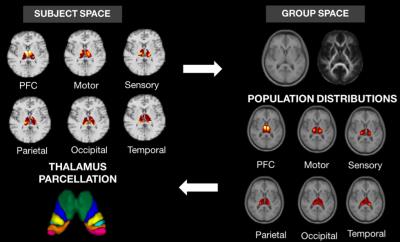
The iterative method (Fig. 1) starts off with initial estimates of probabilistic connectivity maps obtained through tractography. In order to introduce group consistency, a group average space was built through multichannel registration of T1 and FA maps of all normal controls using a cubic B-splines algorithm [5]. Secondly, connection maps from each subject and cortical region were mapped to the group space and averaged. The generated population-based probabilistic connection maps were propagated back to the individual’s space and considered as priors when inferring the new connection probability between every thalamic voxel to a set of cortical regions.
Thalamus parcellation
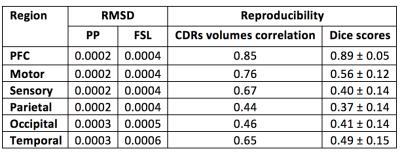
The maximum a posteriori estimates for each region using FSL and the proposed pipeline (PP) were used to classify thalamic voxels. The segmentation performance of both methods were evaluated by estimating the volume fractions of each connectivity-defined regions (CDRs). Underlying group dispersion was quantified through coefficients of variation (CV). Reproducibility of the PP was also tested by replicating the previous steps in two repeated scans, with subsequent comparison of CDRs volumes normalised by the total intracranial volume (TIV), and Dice score between test-retest thalamus parcellations.
Results
Results
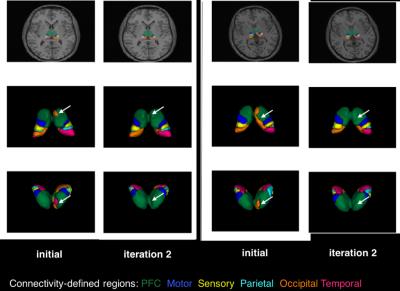
Example thalamus parcellations are shown in Fig. 2. At each iteration, the PP generates smoother probabilistic connectivity maps that led to more anatomically plausible segmentations.
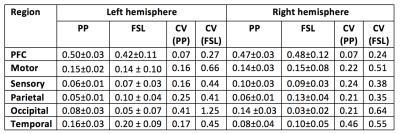
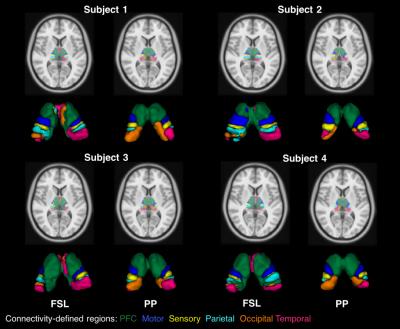
The results generated by PP were compared to FSL. Table 1 describes the relative volumes of each CDR in FSL and proposed strategies. A strong and positive correlation was found between them for both hemispheres (left hemisphere r=0.98, P=0.001; right hemisphere r=0.93, P=0.007). Additionally, CVs from FSL were found to be pronouncedly higher than for the PP, showing a greater dispersion in the normalised volumes across healthy subjects (see Fig.3, where PP provides sharper parcellations).
Under the assumption of left/right symmetry in healthy thalami, the root-mean squared left/right volume differences (RMSD) were measured for each CDR. In this context, a lower RMSD can be seen as an indicator of better left/right symmetry and robustness of the segmentation method (Table 2). In agreement with better appearing parcellations, the PP also led to a lower variability of hemispheric volumes for each thalamus CDR, around 50% less than differences detected in FSL. Test-retest results for the PP are shown in Table 2, with reproducibility being measured by the correlation between test-retest normalised CDRs volumes and test-retest Dice overlap on thalamus parcellations.
Discussion
Discussion
The thalamus clusters (Figs. 2 and 3) demonstrated good correspondence across subjects and with previous studies [8,9].
Anterior clusters were preferentially connected with prefrontal, motor and sensory cortices, while posterior clusters had dominant projections towards parietal, occipital and temporal areas. The proposed method showed greater consistency in anterior than in posterior CDRs (Table 2), likely due to well-known crossing neural pathways. Note that the increased anatomical complexity of the posterior CDRs made the proposed improvements more evident in this region.
Further improvements to the method will involve better acquisition, combining more information when building the group space, as well as a greater number of subjects and pathologies.
Acknowledgements
CS is funded by the Engineering and Physical Sciences Research Council (ESPRC) and Icometrix industrial partner. SBV is funded by the National Institute for Health Research University College London Hospitals Biomedical Research Centre (NIHR BRC UCLH/UCL High Impact Initiative).References
[1] Behrens et al., Nature Neuroscience, 2003; [2] Behrens et al., Elsevier, 2006; [3] NiftTK (http://www.ucl.ac.uk/cmic/software/) [4] Cardoso et al., IEEE TMI, 2015; [5] Modat et al., Computer Methods and Programs in Biomedicine, 2010; [6] Tournier et al., NeuroImage, 2007; [7] Tournier et al., ISMRM, 2010; [8] Johansen-Berg et al., Cerebral Cortex, 2005; [9] Traynor et al., Neuroimage, 2010.Figures