0713
Disrupted Integrity of Frontal White Matter and Neurocognitive Correlates in Patients Treated for Pediatric Medulloblastoma1Diagnostic Imaging, St. Jude Children’s Research Hospital, Memphis, TN, United States, 2Biostatistics, St. Jude Children’s Research Hospital, Memphis, TN, United States, 3Psychology, St. Jude Children’s Research Hospital, Memphis, TN, United States, 4Oncology, St. Jude Children’s Research Hospital, Memphis, TN, United States
Synopsis
This study assessed the longitudinal white matter (WM) microstructure of 146 patients and 72 normal healthy age-similar controls. WM volume, fractional anisotropy (FA), and radial (RAD) and axial (AX) diffusivity trajectories were examined and correlated with neurocognitive performance at 36 months. After surgery but before any additional therapy, frontal WM volume in patients was similar to controls but FA was significantly reduced and was significantly correlated with neurocognitive performance three years later. Over the next three years, WM volume significantly decreased in patients and was significantly correlated with decreased Working Memory.
PURPOSE
In survivors of medulloblastoma, the most common brain tumor in children, decreased white matter (WM) volumes1 and decreased fractional anisotropy (FA) have been observed during and following therapy.2 Effective therapy is also associated with neurocognitive deficits mediated by the frontal lobes, such as working memory,3,4 intelligence,5 processing speed, and attention.4 In this study, we hypothesized that frontal WM would be damaged by disease and/or surgery, resulting in global volumetric changes after therapy. Additionally, we hypothesize that early microstructural changes would be correlated with neurocognitive outcomes after therapy.PATIENTS AND METHODS
Subjects included 146 patients with medulloblastoma, (3.2 to 21.6 years at diagnosis; median=8.7 years), treated with maximal surgical resection, risk-adapted craniospinal irradiation (CSI), and high-dose chemotherapy. Patients with minimal localized disease were assigned to the average-risk (AR) group, while all others were high-risk (HR). MRI examinations were collected at seven time points: baseline (after surgery but before additional therapy); after CSI; and 12, 18, 24, 30, and 36 months after diagnosis. Seventy-two age-similar normal healthy control subjects, (6.0 to 24.5 years at baseline; median=13.0 years), were imaged three times: at baseline, 12 and 24 months. Treatment and imaging protocols were approved by the local Institutional Review Board, and written informed consent was obtained from the patient, subject, parent, or guardian, as appropriate.
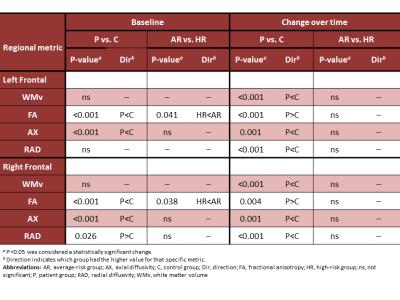
Conventional T1, T2, Proton Density and FLAIR imaging was collected on all subjects using a 1.5T or 3.0T whole-body system (Siemens Medical Systems, Iselin, NJ). These images were registered both within each examination and to the baseline study of each subject before being segmented into CSF, gray and WM.6 Diffusion tensor imaging (DTI), acquired with 12 directions and 4 averages, was processed with the DTI toolkit under SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) to generate maps of FA, radial (RAD) and axial (AX) diffusivity. Seven slices were analyzed and divided into the left and right frontal quadrants. Linear mixed-effects modeling was performed using the restricted maximum–likelihood estimation method to analyze the longitudinal data. Estimates were computed for baseline value, change over time, and interaction between time and subject group (patient vs. control; HR vs. AR vs. control), controlling for age effects using a baseline age term for each subject.
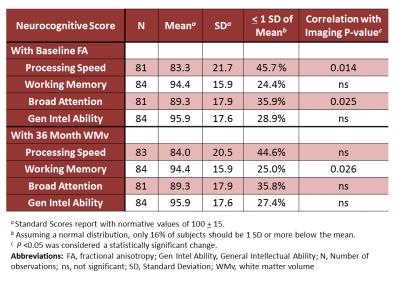
Additionally, the associations between neuropsychological scores 36 months post diagnosis and imaging metrics were explored in a subset of patients with imaging (N=92). Neurocognitive assessments included the Woodcock-Johnson Tests of Cognitive Abilities Third Edition7 to evaluate Processing Speed, Working Memory, Broad Attention, and General Intellectual Ability. Only WM volumes from the 36-month time point and FA from baseline were correlated with these neuropsychological scores. The false discovery rate procedure8 corrected the p-values for multiple testing.
RESULTS
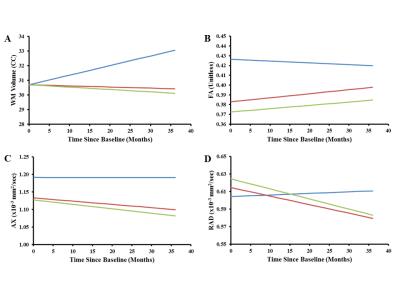
There were a total of 864 patient examinations and 215 control examinations completed. At baseline, WM volumes in patients were similar to those in controls; FA and AX were lower bilaterally (Table 1). Group differences were seen for FA only, where the HR group showed significantly lower FA than the AR group (Figure 1). During follow-up, WM volumes increased in controls but decreased in the patients. FA values in patients increased but never reached control levels. AX and RAD were static in controls but decreased bilaterally in patients.
Decreased WM volume at 36 months was significantly correlated with concurrent decreased Working Memory performance (P=0.026; Table 2). Lower baseline FA was significantly correlated with decreased Processing Speed and Broad Attention at 36 months (p=0.014 and 0.025, respectively) and had trending significance with decreased Working Memory and General Intellectual Ability (p=0.062 for both).
DISCUSSION
Baseline results were consistent with acute, indirect axonal injury caused by disease and/or surgery, which decreased FA and AX but did not appreciably affect WM volume.9 Decreased AX, RAD, and WM volume in follow-up would be consistent with possible resolution of axonal swelling combined with chronic demyelination.9-11 Furthermore, the subsequent near-normalizing increase in FA over the three-year course of treatment and follow-up combined with macroscopic WM volume loss, suggests overall loss of cellular density despite evidence of microstructural recovery.12CONCLUSION
DTI metrics at baseline indicated that patients with medulloblastoma suffer substantial acute microstructural damage to their WM that can be attributed to disease and surgical intervention. This early damage combined with the effects of radiation therapy and chemotherapy was followed by a failure of the WM volume to increase at an age-appropriate rate in patients. While decreased WM volume in survivors is known to be correlated with decreased cognitive performance, this is the first time that decreased FA at baseline, before CSI or chemotherapy, has been shown to be correlated with decreased cognitive performance in survivors.Acknowledgements
We acknowledge the valuable contributions of Rhonda Simmons, advanced signal processing technician and funding in part by the Cancer Center Support Grant P30 CA-21765 from the National Cancer Institute, grant HD049888 from the National Institute of Child Health and Human Development, grant RR029005 from the National Center for Research Resources, and ALSAC.References
1. Reddick WE, Glass JO, Palmer SL, et al. Atypical white matter volume development in children following craniospinal irradiation. Neuro-oncology. 2005; 7(1):12-19.
2. Soelva V, Hernaiz Driever P, Abbushi A, et al. Fronto-cerebellar fiber tractography in pediatric patients following posterior fossa tumor surgery. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2013; 29(4):597-607.
3. Knight SJ, Conklin HM, Palmer SL, et al. Working memory abilities among children treated for medulloblastoma: parent report and child performance. Journal of pediatric psychology. 2014; 39(5):501-511.
4. Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013; 31(28):3494-3500.
5. Schreiber JE, Gurney JG, Palmer SL, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro-oncology. 2014; 16(8):1129-1136.
6. Glass JO, Reddick WE, Reeves C, Pui CH. Improving the segmentation of therapy-induced leukoencephalopathy in children with acute lymphoblastic leukemia using a priori information and a gradient magnitude threshold. Magnetic resonance in medicine. 2004; 52(6):1336-1341.
7. Woodcock R, McGraw K, Mather N. Woodcock-Johnson Third Edition, Tests of Cognitive Abilities. Itasca, IL: Riverside Publishing; 2001.
8. Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995; 57(1):289-300.
9. Aung WY, Mar S, Benzinger TL. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging in medicine. 2013; 5(5):427-440.
10. Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR. American journal of neuroradiology. 2002; 23(5):794-802.
11. Stidworthy MF, Genoud S, Suter U, Mantei N, Franklin RJ. Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain pathology. 2003; 13(3):329-339.
12. van der Voorn JP, Pouwels PJ, Hart AA, et
al. Childhood white matter disorders: quantitative MR imaging and spectroscopy.
Radiology. 2006; 241(2):510-517.
Figures