0712
Differentiating Recurrent Glioma from Treatment Effects Using Amide Proton Transfer-Weighted MRI1Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
We assess the feasibility and the value of amide proton transfer-weighted (APTw) MRI in identifying viable malignant glioma. 22 patients with suspected recurrent glioma following chemoradiation were scanned at 3T. A total of 64 stereotactic biopsy specimens were obtained from gadolinium-enhancing regions of interest with varying APTw signals, 47 of which were histopathologically assigned as recurrent tumor and 17 treatment effects. APTw MRI revealed different signal intensities in the biopsied sites showing recurrent tumor (hyperintense signal) or treatment effects (
Clinical Question
Standard neuroimaging lacks sufficient specificity for the assessment of glioma response to therapy.Impact
MR imaging is a standard modality for neuroimaging. However, this modality, based primarily on gadolinium (Gd) enhancement (a marker of blood-brain-barrier disruption), has become more and more challenging. For example, Gd-enhanced MRI cannot distinguish between tumor recurrence and treatment effects (such as radiation necrosis and pseudoprogression1,2), because both are associated with blood-brain barrier disruption (thus Gd enhancement). The "pseudoprogression" phenomenon can be observed in 20-30% of patients within the first several months after standard temozolomide (TMZ) chemoradiation therapy. Conversely, when antiangiogenic therapies are used, Gd enhancement may disappear immediately after initiating therapy, and tumor recurrence often appears as a nonenhancing tumor (called “pseudoresponse”).3 Currently, pseudoprogression and pseudoresponse have been two new formidable diagnostic dilemmas in neuro-oncology.4,5Approach
Traditional approach
The standard clinical MRI sequences include T2w, FLAIR, T1w, and Gd- T1w. There are numerous ongoing investigations into the ability of functional and molecular imaging techniques to assess treatment effects.6 However, there is currently no standard imaging modality available for differentiating between true tumor progression and treatment effects in the clinic. Presently, post-treatment patients with suspected recurrence are often referred for repeat surgery to obtain pathologic confirmation of recurrent cancer, given the limitations of current MRI sequences.7
New APTw Imaging Approach
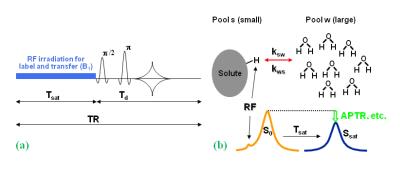
Amide proton transfer-weighted (APTw) imaging (Fig. 1) is a novel protein-based molecular MRI technique that can provide contrast due to endogenous mobile proteins and peptides and tissue pH.8 Numerous early clinical APTw MRI studies have shown promising diagnostic value in tumor grading9-11 and differentiating between tumor and peritumoral edema.12 Our preclinical study in rats clearly showed that untreated glioma (hyperintense) and radiation necrosis (hypointense or isointense) exhibited opposite APTw signals, and could, thus, readily be distinguished.13 In this study, we hypothesized that APTw MRI would specify regions of recurrent tumor in a heterogeneous background, which might serve as a diagnostic tool for the sensitive and specific identification of recurrent malignant glioma.
Gains and Losses
Histopathology diagnosis via biopsied tissue is often needed for the diagnosis of recurrent malignant glioma. However, due to tremendous heterogeneity, choosing a representative area for tissue sampling can be very challenging, and pathology results may still be variable.14 The incidence of unsuccessful biopsies (nonrepresentative or nondiagnostic specimens) is approximately 8%.15 The APTw imaging signal as an effective surrogate biomarker of recurrent glioma in patients could be used to guide a biopsy that could greatly increase the accuracy of pathology. Further, the biopsy approach is invasive. It has been reported that ~6% of patients who underwent surgical biopsy suffered severe complications, such as increased neurological deficits, and ~2% of patients died following the procedure.16 APT MRI has the potential to enhance the noninvasive molecular diagnosis of brain tumors before and after treatment. This could potentially reduce the need of repeated biopsies and their associated risks of complications, thereby improving the quality of life for patients and decreasing the cost of care. It is worthwhile to mention that APTw MRI can be accomplished without exposure to Gd contrast agents, which has been indicated as a risk for patients with kidney dysfunction and for any patients due to potential brain deposits.Preliminary Data
MRI and Histopathologic Features
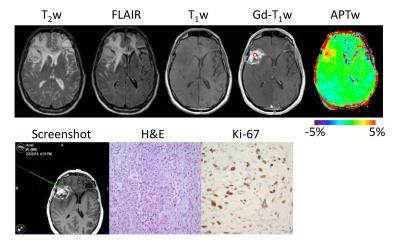
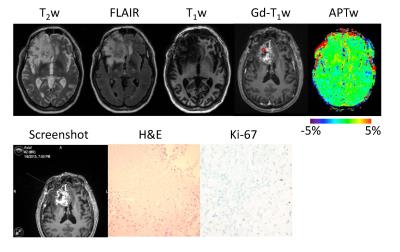
22 patients with suspected recurrent malignant glioma following chemoradiation were recruited and underwent a volumetric APTw imaging sequence at 3T. A total of 64 stereotactic biopsy specimens were obtained from Gd-enhancing regions of interest with varying APTw signals. Although these biopsied regions shared similar radiographic characteristics on the routine MRI images, the APTw images revealed different characteristics among the different lesions (Figs. 2-10). In lesions that harbored recurrent tumor (Fig. 2), the APTw images were characteristically nodular and heterogeneous with intermediate to substantial hyperintensity, compared to the CNAWM. In contrast, in lesions that corresponded to treatment effects (Fig. 3), the APTw images exhibited relatively homogeneous iso-intensity with a minimal amount of scattered punctate hyperintensity.
Quantitative Analysis of APTw Signal Intensities
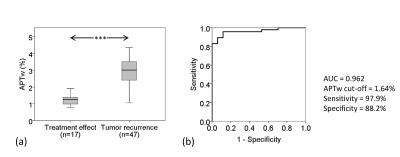
For all 64 biopsied specimens (Fig. 11), the mean APTw intensities at lesions that represented recurrent tumor were significantly different from lesions that represented treatment effects (P < 0.001). ROC curve analysis showed that, for the differentiation of recurrent tumor from treatment effects, APTw MRI had an area under the curve (AUC) of 0.962, with a 97.9% sensitively and an 88.2% specificity at the cutoff APTw intensity of 1.64%, compared the conventional Gd-T1w sequence approximately with a 94.1% sensitively and a 50.0% specificity.
Acknowledgements
The authors thank our neurologists (Drs. Jaishri O. Blakeley, John Laterra, Matthias Holdhoff), neurosurgeons (Drs. Michael Lim, Alfredo Quinones-Hinojosa, Jon D. Weingart), neuropathologists (Drs. Charles G. Eberhart and Huamin Qin), neuroradiologists (Zhibo Wen, Martin G. Pomper), biostatistician (Dr. Peng Huang), MRI physicist (Dr. Peter B. Barker), and research nurse (Ms. Lindsay Blair) for their cooperation on this project. This work was supported in part by grants from the NIH (EB009731, CA166171, EB015032, and EB015909).References
1. Brandes, A.A., et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 26, 2192-2197 (2008).
2. Brandsma, D., Stalpers, L., Taal, W., Sminia, P. & van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 9, 453-461 (2008).
3. Pope, W.B., Lai, A., Nghiemphu, P., Mischel, P. & Cloughesy, T.F. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 66, 1258-1260 (2006).
4. Clarke, J.L. & Chang, S. Pseudoprogression and pseudoresponse: Challenges in brain tumor imaging. Curr. Neurol. Neurosci. Rep. 9, 241-246 (2009).
5. Wen, P.Y., et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 28, 1963-1972 (2010).
6. Verma, N., Cowperthwaite, M.C., Burnett, M.G. & Markey, M.K. Differentiating tumor recurrence from treatment necrosis: a review of neurooncologic imaging strategies. Neuro-Oncology 15, 515-534 (2013).
7. Woodworth, G.F., et al. Histopathological correlates with survival in reoperated glioblastomas. J. Neuro-Oncol. 113, 485-493 (2013).
8. Zhou, J., Payen, J., Wilson, D.A., Traystman, R.J. & van Zijl, P.C.M. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature Med. 9, 1085-1090 (2003).
9. Zhou, J., et al. Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J. Magn. Reson. Imaging 38, 1119-1128 (2013).
10. Togao, O., et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-Oncology 16, 441-448 (2014).
11. Sakata, A., et al. Grading glial tumors with amide proton transfer MR imaging: different analytical approaches. J. Neuro-Oncol. 122, 339-348 (2015).
12. Wen, Z., et al. MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. NeuroImage 51, 616-622 (2010).
13. Zhou, J., et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nature Med. 17, 130-134 (2011).
14. Melguizo-Gavilanes, I., Bruner, J.M., Guha-Thakurta, N., Hess, K.R. & Puduvalli, V.K. Characterization of pseudoprogression in patients with glioblastoma: is histology the gold standard? J. Neuro-Oncol. 123, 141-150 (2015).
15. Soo, T.M., et al. Failed stereotactic biopsy in a series of 518 cases. Stereotact Funct Neurosurg 64, 183-196 (1995).
16. Bernstein, M. & Parrent, A.G. Complications of CT-guided stereotactic biopsy of intra-axial brain lesions. J Neurosurg 81, 165-168 (1994).
Figures