0680
Prospective motion correction for 3D GRASE pCASL with volumetric navigators1Laboratory of FMRI Technology (LOFT), Mark & Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Radiology, Harvard Medical School, Boston, MA, United States
Synopsis
We propose a prospective motion correction approach for background suppressed (BS) segmented 3D GRASE pCASL using volumetric EPI-based navigators (vNavs), which causes minimal contrast change and no extra time. vNavs reduced motion artifacts effectively and increased temporal signal-to-noise ratio (t-SNR). Principle component analysis (PCA) is able to further reduce residual motion artifacts and restore the details of gyral structure in perfusion weighted images.
Purpose
Segmented 3D acquisition was recently recommended as the preferred method for arterial spin labeling (ASL) scanning, due to its relatively high signal-to-noise ratio (SNR) [1]. Segmented 3D readout reduces spatial blurring due to T2 relaxation, however it makes 3D ASL sensitive to head motion, which introduces ghosting artifacts in label/control images and is challenging for retrospective motion correction. In this study, we propose a prospective motion correction approach for background suppressed (BS) segmented 3D GRASE pCASL using volumetric EPI-based navigators (vNavs) for inline inter-segmentation motion correction [2].Methods
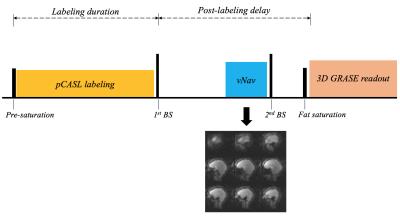
As demonstrated in Fig. 1, vNavs were inserted before the second background suppression pulse within the post labeling delay (PLD). One low-resolution 3D EPI volume was acquired from the navigator and registered to the reference volume to update the FOV of the following GRASE readout. Experiments were performed on a Siemens 3T Skyra scanner (Erlangen, Germany) using a 20-channel head coil. The subjected was scanned with and without deliberate motion, which was prompted approximately once a minute.
Imaging parameters for GRASE pCASL were: FOV=280 mm, 40 slices with 20% slice oversampling, resolution=2.9×2.9×3 mm3, bandwidth=2264 Hz/pixel, echo spacing=0.51 ms, TE=37.76 ms, TR=3760 ms, label/control duration=1500 ms, Post-labeling delay (PLD) = 1800 ms, turbo factor=8, segments=6 and total scan time is 5 min 40 sec. Imaging parameters for vNav were: flip angle=2°, resolution=8×8×8 mm3, matrix size=32×32×32, 6/8 partial Fourier along partition, bandwidth=4734 Hz/pixel, TE=5 ms, TR=11 ms, and total acquisition time of 275 ms. Temporal SNR (t-SNR) was calculated as the mean divided by the standard deviation of perfusion time series within gray matter (GM). Acquired images were also aligned to the first volume by rigid motion correction using SPM in Matlab.
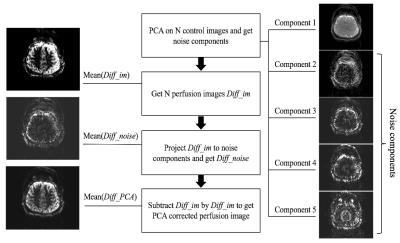
Though vNavs showed high accuracy in detecting and correcting rigid head motion between segments, motion within each segment cannot be corrected with this method. To address intra-segment motion, principle component analysis (PCA), a statistical procedure separating the image series into an orthogonal set of components while specific components are considered as noise, was performed offline [3], as demonstrated in Figure 2.
Results and discussion
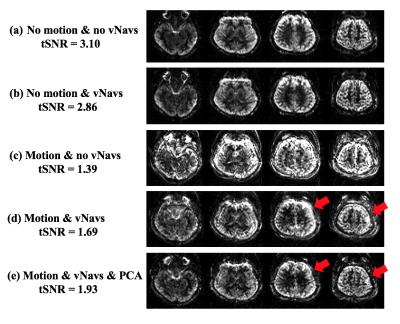
Figure 3 shows four representative slices of perfusion images acquired with and without vNavs, under both motion conditions respectively (with and without intentional head motion). Navigators caused minimal contrast change and produced similar tSNR in reference scans, as shown in Figure 3 (a) and (b). Under motion conditions, Figure 3 (d) shows that vNavs reduced motion artifacts and increased tSNR from 1.39 to 1.69 compared to the scan acquired without vNavs. Figure 3 (e) indicates that PCA is able to further reduce motion artifact and restore the details of gyral structure in perfusion weighted images, as indicated by red arrows. With motion detected by vNavs, reacquisition is an option in the future to compensate the measurements with severe motion and further increase t-SNR at the cost of increased scan time.Conclusion
Prospective motion correction using vNavs was implemented and suppressed motion artifacts effectively for segmented 3D GRASE pCASL. Residual motion artifacts were compensated with PCA.Acknowledgements
This work was supported by NIH R00HD074649, R21AG046657, R01HD071664 and R01HD085813.References
[1] Alsop D C, et al. MRM, 2015, 73(1): 102-116. [2] Tisdall, et al. MRM, 2012, 68:389-99, 2012. [3] Lin W, et al. MRM, 2008, 60(5): 1135-1146.Figures