0677
Evaluation of Velocity Selective ASL Tagging Efficiency Utilizing Accelerated 3D Angiography1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Velocity Selective Arterial Spin Labeling (VS-ASL) has been suggested as a possible solution for evaluating perfusion and MRA in complex geometries without well-defined labeling planes as well as in cases of slow or complex flow patterns. The goal of this work is to assess the spatial distribution of VS-ASL tagging efficiency utilizing MR angiography. Specifically, we identify and characterize errors introduced by susceptibility induced magnetic gradients. In controlled phantom experiments and human subjects tagging efficiency demonstrated dependence both on the magnitude of susceptibility shift and the velocity of vessels flowing through those regions.
Purpose
Velocity Selective Arterial Spin Labeling (VS-ASL) is a promising technique for transit time insensitive, non-contrast perfusion and vascular imaging [1-3]. In contrast to alternative ASL techniques, the tagging module is targeted to be velocity rather spatially selective. Thus the labeling can be effective in cases of complex geometries without well-defined labeling planes or in slow or complex flow patterns. However, VS-ASL tagging is subject to other error sources including eddy currents, B1 heterogeneity, subject motion, and/or diffusion sensitization. The goal of this work is to assess the spatial distribution of VS-ASL tagging efficiency utilizing accelerated MRA imaging. Specifically, we examine the effect of gradients introduced by local changes in susceptibility in both controlled phantom experiments and human subjects.Methods
Eddy current and B1 insensitive tagging has been previously demonstrated using a symmetric, adiabatic BIR-8 preparation module [4]; where images are acquired with and without flow sensitizing gradients. Experiments were performed to investigate the impact of local susceptibility induced gradients for this preparation module. This is especially relevant in the cerebrovasculature as the delivering arteries flow along the base of the brain in the proximity of the nasal cavity.
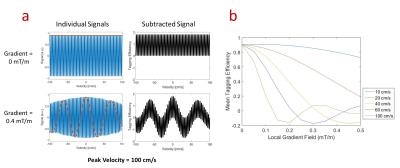
Simulations: Bloch simulations of steady, parabolic flow were used to evaluate the impact of local susceptibility gradients on the mean tagging efficiency of blood with a Venc of 2 cm/s and background gradients ranging from 0 to 0.1 mT/m. Tagging efficiency was defined as the mean difference between signal with and without flow sensitizing gradients.
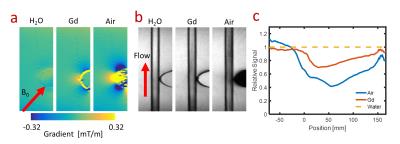
Phantoms: Imaging was performed on a 3T MR system (MR750, GE Healthcare, Waukesha, WI). A phantom was constructed with a 6 mm tube oriented 45° with respect to main magnetic field and an adjacent susceptibility insert. The insert allowed for interchange between air, 20 mM/mL Gd (Multihance, Bracco Diagnostics, NJ), and water. For each susceptibility insert, images were collected with and without flow at a peak velocity of ~50 cm/s.
Volunteers:
Normal volunteers were
imaged for this IRB approved, HIPAA compliant study. ASL preparation pulses
were played out followed by either a Cartesian fast spin echo acquisition for
perfusion imaging or a radial gradient echo acquisition for MRA. The perfusion
sequence included a labeling delay time of 1.5 s. MRA images were collected
utilizing no labeling delay before acquisition of 77 individual radial lines using
a variable sampling density in the readout direction [5] followed by a 2 s
delay to allow for T1 recovery. Labeling and control pulses were interleaved to
reduce sensitivity to bulk motion. Imaging was performed using the optimal
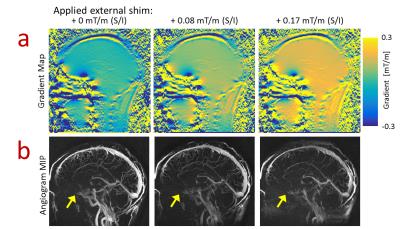
system shims followed by increasing the shim in the volunteer's superior/inferior
(S/I) direction to perturb the gradient field over the labeling volume. Tagging
efficiency was determined in vivo using: efficiency = (control signal / tag
signal)/proton density
Results
Simulations: Individual signal profiles (Figure 1a) and the mean tagging efficiency (Figure 1b) in parabolic flow show reduced efficiency with static gradients. In particular, signal loss in the control images manifests when the static gradient and velocity are both high.
Phantoms: This result is corroborated in phantom experiments (Figure 2). Control images show signal loss in the water flowing past the inserts with greater susceptibility resulting in a lower tagging efficiency in subtracted images. During the 250 ms readout this signal loss is propagated downstream.
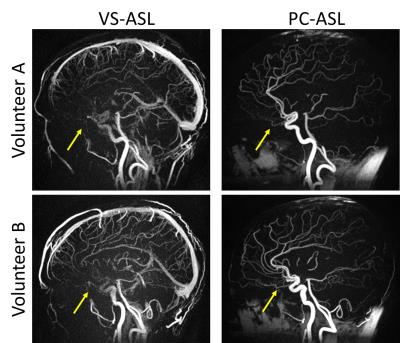
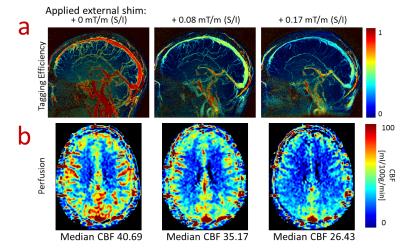
Volunteers: MRA images in volunteers demonstrate the ability to visualize both arterial and venous structures using VS-ASL as well as local signal loss in the sinus (Figure 3) demonstrating the impact of tagging the moving spins at the location of local gradients. MRA images with no tagging delay show signal dropout in the arteries flowing adjacent to the sinus at all shim settings while all regions with rapid flow show decreased signal as the external shim was increased (Figure 4) which is corroborated with the tagging efficiency maps (Figure 5a). Finally, perfusion maps show decreased perfusion in regions fed by rapid flow with greater external shim (Figure 5b).
Discussion and Conclusions
VS-ASL approaches have been shown to provide advantages over other techniques; including our own MRA results demonstrating this method may be used to image venous structures. However this work also demonstrates decreases in the mean tagging efficiency that were found to be dependent on the peak velocity in blood vessels and local magnetic field gradients. This was shown to lead to decreased perfusion signal as well as drop-outs in vessels for MRA imaging and should be considered when utilizing these methods. Care should be taken when utilizing VS-ASL and the effect of static gradients should be weighed against other artifacts when designing tagging modules.Acknowledgements
The authors wish to acknowledge NIH R01NS066982 and GE Healthcare for their research support.References
[1] Wong EC, Cronin M, Wu W-C, Inglis B, Frank LR, Liu TT. Velocity selective arterial spin labeling. Magn Reson Med 2006;55:1334–1341.
[2] Fan Z, Sheehan J, Bi X,Liu X, Carr J, Li D. 3D Noncontrast MR Angiography of the Distal Lower Extremities Using Flow-Sensitive Dephasing (FSD)-Prepared Balanced SSFP. Magn Reson Med 2009;62:1523-1532
[3] Zhang N, Fan Z, Luo N, Bi X, Zhao Y, An J, Liu J, Chen Z, Fan Z, Li D. Noncontrast MR Angiography (MRA) of Infragenual Arteries Using Flow-Sensitive Dephasing (FSD)-Prepared Steady-State Free Precession (SSFP) at 3.0 Tesla:Comparison with Contrast-Enhanced MRA. J Magn Reson Imaging 2016;43:364-372
[4] Guo J, Meakin JA, Jezzard P, Wong EC. An Optimized Design to Reduce Eddy Current Sensitivity in Velocity-Selective Arterial Spin Labeling Using Symmetric BIR-8 Pulses. Magn Reson Med 2015;73:1085-1094
[5] Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D Ultrashort Echo Time Pulmonary MRI. Magn Reson Med 2013;70:1241-1250
Figures