0669
Deep learning to improve prostate cancer diagnosis1Electrical Engineering, University of Surrey, Guildford, United Kingdom, 2Centre for Medical Imaging, University College London, 3Histopathology, University College London, 4Surgery and Interventional Science, University College London
Synopsis
Multiparametric MRI (mp-MRI) can localize tumour within the prostate, guide biopsy, and assess disease burden. Nevertheless, mp-MRI itself remains imperfect. Almost 40% of mp-MRI studies are reported as indeterminate for significant cancer. An indeterminate mp-MRI confers no patient benefit, such patients require either repeat interval mp-MRI and/or subsequent biopsy. There remains a clear unmet need to improve diagnostic imaging over and above standard mp-MRI protocols. Previous work has developed zone specific logistic regression (LR) models for the determination of significant cancer (any cancer-core-length (CCL) with Gleason>3+3 or any grade with CCL≥4 mm) in the peripheral (PZ) and the transition zone (TZ) based on quantitative mp-MRI parameters following MR imaging. This work proposes a state-of-art deep learning method to detect prostate cancer both in the PZ and the TZ. The proposed model is trained on a cohort of patients imaged at a 3T scanner, and validated on independent cohort of patients imaged at a 1.5T scanner. The performance of the model was compared with LR, Support Vector Machines and traditional Neural Networks.
Purpose
This study determines the ability of deep learning to improve the detection of significant prostate cancer in the peripheral (PZ) and the transition (TZ) zone using quantitative multiparametric MRI (mp-MRI). Specifically a deep belief network (DBN) model was trained on a cohort of patients imaged at a 3T scanner, and validated on two independent cohort of patients imaged at a 1.5T scanner. The performance of the DBN model was compared against traditional multilayer perceptron neural networks (MLP), support vector machines (SVM) and logistic regression (LR) in terms of accuracy and generalization.
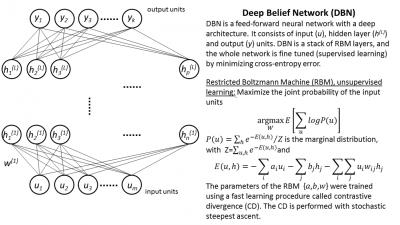
Deep Belief Networks
DBN consists of a greedy layer-wise unsupervised pre-training followed by supervised fine-tuning [1]. In this work each layer was pre-trained using a Restricted Boltzman Machine (RBM) unsupervised learning algorithm [2]. DBN involves training several RBMs and is computationally expensive. At the final training phase the whole deep architecture is fine tuned using back-propagation. The deep structure of DBN is essential to learn complex functions [3], and the extra pre-training step (unsupervised learning) avoids local minima due to the backpropagation step. Figure 1 shows an overview of the DBN algorithm.
Methods
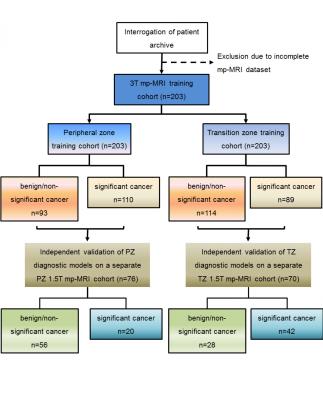
Three hundred and forty-nine patients (203 training cohort; 76 PZ independent cohort; 76 TZ independent cohort) underwent mp-MRI and transperineal template prostate mapping biopsy. All patients within the training cohort underwent a 3T (Achieva, Philips Medical Systems, Best, Netherlands) mp-MRI. All images were acquired with a pelvic-phased array coil; 0.2 mg/kg (maximum 20 mg) of spasmolytic (Buscopan; Boehringer Ingelheim, Ingelheim, Germany) was administered intravenously to reduce peristalsis. The mp-MRI included axial and coronal small field of view T2-weighted imaging; and was supplemented by axial diffusion weighted imaging (DWI) and dynamic contrast enhanced (DCE) imaging as reported in the PICTURE dataset [4]. mp-MR images were re-analyzed with MIM Symphony Version 6.1 (MIM Software Inc, Cleveland, USA). Two board certified radiologists in consensus (10 and 4 years experience), aware of the original pre-biopsy mp-MRI report (produced as part of the PICTURE study) and the histological findings manually contoured regions of interest (ROI) on mp-MRI (T2-weighted, ADC maps, and contrast early-enhanced T1-weighted images). One PZ and one TZ location was contoured per patient. Locations containing significant prostate cancer were deemed positive. The mean SI of each ROI on corresponding T2-weighted images, ADC maps, and early arterial contrast-enhanced images was recorded. T2 and early T1 arterial contrast enhanced SI was normalized against a right obturator internus ROI to derive a SI ratio, to determine normalized T2 (T2-nSI) and normalized early contrast-enhanced T1 SI (DCE-nSI) parameters. Maximum enhancement (ME) was calculated from the DCE signal enhancement time curve as previously reported [5]. Previous work [5, 6] has showed that the three best classifiers for TZ prostate cancer are ADC, T2-nSI and ME, and for PZ cancer are ADC, T2-nSI and DCE-nSI. PZ and TZ prostate cancer diagnostic models were trained based on the previously found best classifiers, and were independently validated on the previously published PZ and TZ cohorts of patients imaged at a 1.5T (Avanto, Siemens, Erlangen, Germany) scanner [5]. The examined diagnostic models were validated using receiver operating characteristic (ROC) analysis. Figure 2 shows a flowchart of the study.
Results
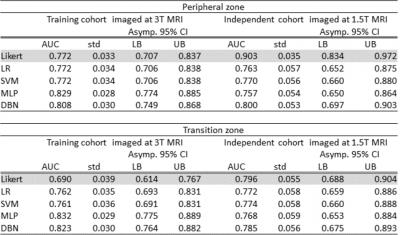
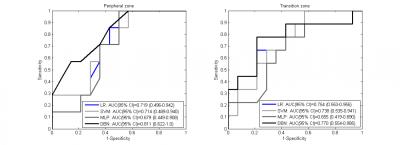
Table 1 shows the ROC-AUC for the detection of PZ and TZ significant cancer of the validated models (LR, SVM, MLP, DBN) in the training (3T MRI) and the independent (1.5T MRI) cohorts of patients. For the cases who were scored as indeterminate (Likert 3/5) by the radiologist the zone-specific DBN performed better than the other models, demonstrating a ROC-AUC=0.811 (%CI: 0.622-1.0) in the PZ and an ROC-AUC= 0.770 (%CI: 0.554-0.985) in the TZ. The sensitivities for 50% specificity were 85.7% (LR), 85.7% (SVM), 100% (MLP), 92.9% (DBN) in the PZ and 77.8% (LR), 88.9% (SVM), 77.8% (MLP), 88.9% (DBN) in the TZ. The respective ROCs are shown in figure 3.
Conclusions
DBN outperformed the other models in terms of AUC and it's performance did not drop significantly when applied to an independent cohort of patients. MLP as expected performed slightly better in the training cohorts, but dropped significantly in the independent cohort. This finding confirms that the generalization performance of MLP is worse than DBN. Compared to an experienced radiologist, when the DBN model was applied on the independent cohort it performed similarly in the TZ and worse in the PZ. Nonetheless for the indeterminate areas by the radiologist, DBN exhibited high AUC and could potentially aid in their classification.Acknowledgements
This work was undertaken at the centre for vision, speech and signal processing (CVSSP), university of Surrey and UCLH/UCL, which received a proportion of the funding from the NIHR Biomedical Research Centres funding scheme of the UK Department of Health. The work of HSS and SP was supported by the KCL/UCL Comprehensive Cancer Imaging Centre.
References
[1] Hinton GE, Osindero S, Teh YW. A fast learning algorithm for deep belief nets. Neural computation. 2006; 18(7):1527–1554.
[2] Tanaka M and Okutomi M, A Novel Inference of a Restricted Boltzmann Machine, International Conference on Pattern Recognition (ICPR2014), 2014.
[3] Bengio Y. Learning deep architectures for AI.Foundations and Trends in Machine Learning. 2009: 2(1):1–127.
[4] Simmons LA, Ahmed HU, Moore CM, et al. The PICTURE study -- prostate imaging (multi-parametric MRI and Prostate HistoScanning™) compared to transperineal ultrasound guided biopsy for significant prostate cancer risk evaluation. Contemp Clin Trials. 2014;37(1):69-83.
[5] Dikaios N, Alkalbani J, Sidhu HS, et al. Logistic regression model for diagnosis of transition zone prostate cancer on multi-parametric MRI. Eur Radiol. 2015; 25(2):523-32.
[6] Dikaios N, Alkalbani J, Abd-Alazeez M, et al. Zone-specific logistic regression models improve classification of prostate cancer on multi-parametric MRI. Eur Radiol. 2015; 25(9):2727-37.
Figures