0662
3D MR Elastograpy in Prediction of Tumor Capsule Formation of Hepatocellular Carcinoma (HCC) in Patients with Hepatitis B Virus Infection1Department of Radiology, the Third Affiliated Hospital, Sun Yat-sen University (SYSU), Guangzhou, People's Republic of China, 2Department of Pathology, the Third Affiliated Hospital, Sun Yat-sen University (SYSU), Guangzhou, People's Republic of China, 3Department of Radiology, Mayo Clinic, Rochester, United States, 4GE Healthcare MR Research China, Guangzhou, People's Republic of China
Synopsis
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related deaths around the world. Tumor capsule formation is a significant and independent predictor of survival and recurrence. We explored the potential value of HCC stiffness using 3D MR elastograpy for the prediction of tumor capsule formation. Results in 50 examinations showed that HCC stiffness has promise for the preoperative prediction of tumor capsule formation, thus providing motivation for further evaluation of HCC characteristics with MRE.
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer deaths worldwide and recurs in approximately 70% of cases after resection1-3. Tumor capsule formation can be classified as gross or microscopic and is a significant and independent predictive factor of overall survival and recurrence, even in small HCCs4-6. MR imaging plays an important role in the diagnosis and management of patients with HCC, though sometimes conventional MRI techniques fail to demonstrate very thin (0.1-0.3 mm)HCC tumor capsules and HCCs are considered negative for tumor capsule in pathology reports even though dynamic MR imaging clearly demonstrated an enhancing rim on delayed-phase images7-11. MR elastography (MRE) is a method for measuring the mechanical properties of tissue, such as stiffness, that has shown promise for distinguishing HCC from benign tumors and predicting the prognosis of chronic liver diseases12-15. The aim of this study was to evaluate the potential utility of tumor stiffness for predicting tumor capsule formation.Methods
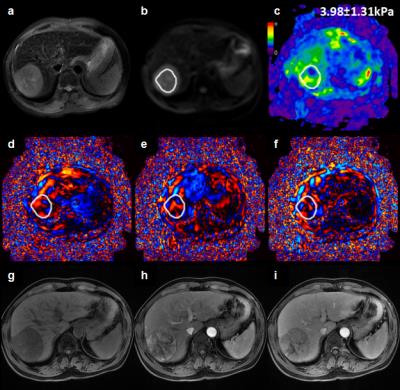
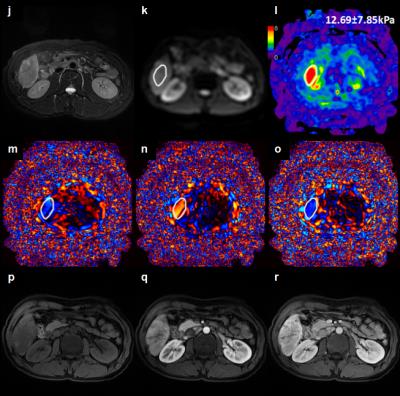
96 patients with HCC who underwent surgical resections and a preoperative three-dimensional (3D) MRE examination between December 2014 and September 2016 were identified for this study. 46 patients were excluded because their tumor was small (<20 mm), the MRE images did not include the HCC, or the MRE information in the HCC was unsatisfactory for analysis. The final study group consisted of 50patients(41 males, 9 females; mean age: 48.67±10.08 years, age range: 24–72 years; mean body mass index (BMI): 22.37±3.42kg/m2; BMI range: 16.28–30.49kg/m2)with a mean HCC diameter of 67.53±34.72mm(range: 29-190mm). Liver biopsy specimens were reviewed for tumor capsule formation by an experienced hepatopathologist who was blinded to the clinical patient data and MRE results. Tumor staging and histological grade were evaluated. HCCs were classified into two groups: group 1 = tumor capsule present, group 2 = tumor capsule absent. MRE was performed within one month prior to surgical resection. MRE was performed on a 3.0T scanner with a SE-based multislice EPI 3D-MRE sequence at 60Hz (acquisition matrix: 80x80; TR/TE:1334/52ms;single shot; scan time:1:04 min; FOV:44.8 cm; number of slices: 20; slice thickness: 3mm). The MRE magnitude and phase images were processed using a direct inversion algorithm (DI) to calculate the stiffness16. In patients with multiple tumors, the largest one was analyzed. Regions of interest(ROIs) were drawn manually tracing the HCC on every slice demonstrating the tumor and covering the entire HCC on the magnitude images with T2-weighted images as a reference. The ROIs excluded tumor edges, areas of significant wave interference and any other artifacts seen on the magnitude and phase images. The mean stiffness of the HCC in kilopascals (kPa) was calculated. The Student's t test was used to compare the AFP, tumor size and mean tumor stiffness, and the chi-squared test was performed to compare the tumor staging and histological grade between two groups. Statistical significance was defined as P<0.05.Results
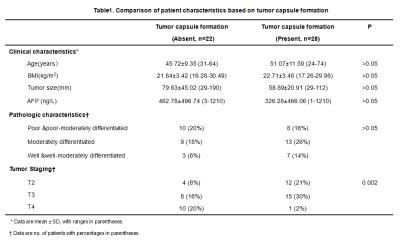
At histology 28HCCs(56%) had evidence of tumor capsule formation and 22 HCCs (44%) did not have any tumor capsule formation. Comparisons of patient clinicopathological characteristics according to presence or absence of tumor capsule formation are shown in Table 1. Baseline variables, including age, sex, BMI, serum AFP and tumor size, were similar in both groups(P>0.05). However, there was a significant difference in tumor staging between the two groups(P=0.002).The mean tumor stiffness of tumor was 4.60±1.13kPa(range: 2.98-7.50kPa) with tumor capsule formation.(Figure 1) ,and 6.73±2.75kPa(range: 2.91-12.69kPa) in tumors without tumor capsule formation(Figure 2) There was a significant difference in the mean stiffness value(P=0.004).Discussion
Our study results showed that there was a significant difference in tumor staging between HCCs without tumor capsule formation compared to those with tumor capsule formation. Tumor stiffness was significantly lower in HCCs with tumor capsule compared to those without tumor capsule. The invasive HCCs (without tumor capsule) tend to be present at advanced tumor stages and they show higher stiffness. Our study demonstrated that MRE has the potential to assess the tumor capsule formation of HCC preoperatively and noninvasively. Further development of this technique is warranted.Conclusion
3D MR elastography is a promising noninvasive technique for predicting the tumor capsule formation of HBV-related HCCs preoperatively. Use of MRE may lead to new quantitative tissue characterization parameters for reflecting HCC biological properties and heterogeneity.Acknowledgements
No acknowledgement found.References
1.de Martel C, Maucort-Boulch D, Plummer M, et al. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology,2015,62(4):1190-1200.2.
2.Colecchia A, Schiumerini R, Cucchetti A, et al. Prognostic factors for hepatocellular carcinoma recurrence. World J Gastroenterol, 2014,20:5935-5950.
3. Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocelluar carcinoma. Gastroenterology. 2009, 137(3): 850-855.
4. Laurent C, Blanc JF, Nobili S, et al. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J Am Coll Surg. 2005, 201(5):656-62.
5. Abdel-Wahab M, El-Husseiny TS, El Hanafy E, et al.Prognostic factors affecting survival and recurrence after hepatic resection for hepatocellular carcinoma in cirrhotic liver. Langenbecks Arch Surg. 2010, 395(6):625-326.
6. Takeishi K, Maeda T, Shirabe K, et al. Clinicopathologic features and outcomes of non-B, non-C hepatocellular carcinoma after hepatectomy. Ann SurgOncol. 2015, 22 Suppl 3:S1116-24.7.
7. Choi YS, Rhee H, Choi JY, et al. Histological characteristics of small hepatocellular carcinomas showing atypical enhancement patterns on gadoxetic acid-enhanced MR imaging.J MagnReson Imaging. 2013, 37(6):1384-91.
8.Davarpanah AH, Weinreb JC.The role of imaging in hepatocellular carcinoma: the present and future. J Clin Gastroenterol. 2013, 47 Suppl:S7-10. doi: 10.1097/MCG.0b013e31827f0d3d.
9.Xu P, Zeng M, Liu K, et al.Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging?J GastroenterolHepatol. 2014, 29(2):330-6. doi: 10.1111/jgh.12358.
10. Grazioli L, Olivetti L, Fugazzola C, et al. The pseudocapsule in hepatocellular carcinoma: correlation between dynamic MR imaging and pathology. Eur Radiol, 1999, 9(1): 62-7.
11. Ishigami K, Yoshimitsu K, Nishihara Y, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology. 2009, 250(2): 435-43.
12. Lee DH, Lee JM, Yi NJ, et al. Hepatic stiffness measurement by using MR elastography: prognostic values after hepatic resection for hepatocellular carcinoma. EurRadiol. 2016 Jul 25. [Epub ahead of print]
13. Hennedige TP, Hallinan JT, Leung FP, et al. Comparison of magnetic resonance elastography and diffusion-weighted imaging for differentiating benign and malignant liver lesions. EurRadiol. 2016;26(2):398-406.
14. Venkatesh SK, Yin M, Glockner JF, et al.MR elastography of liver tumors: preliminary results.AJR Am J Roentgenol. 2008, 190(6):1534-40.
15. Morisaka H, Motosugi U, Glaser KJ, et al. Comparison of diagnostic accuracies of two- and three-dimensional MR elastography of the liver. J MagnReson Imaging. 2016 Sep 23.doi: 10.1002/jmri.25425. [Epub ahead of print]
16. Shi Y, Glaser KJ, Venkatesh SK, et al., Feasibility of using 3D MR elastography to determine pancreatic stiffness in healthy volunteers. J Magn Reson Imaging. 2015;41(2):369-75.
Figures