0656
New insights into the predilection sites of Juvenile Osteochondritis Dissecans using Quantitative Susceptibility Mapping1Radiology, University of Minnesota, Minneapolis, MN, United States, 2Radiology, CMRR, University of Minnesota, 3University of Minnesota, 4College of Veterinary Medicine, University of Minnesota, 5St. Lukes Orthopaedics, Boise, ID, 6College of Veterinary Medicine, University of Minnesota, St. Paul, MN, 7Department of Applied Physics, University of Eastern Finland, 8Diagnostic Imaging Center, Kuopio University Hospital, Kuopio, Finland
Synopsis
The unique utilization of tissue-inherent MRI contrast using QSM for depicting the intraepiphyseal vascular supply provides high resolution and accuracy without using exogenous contrast making it a noninvasive tool for future in vivo studies. A better understanding of the development of the epiphyseal cartilage vascularity during skeletal maturation may shed light on the etiology of many developmental diseases, such as Juvenile Osteochondritis Dissecans that are precursors to osteoarthritis.
Introduction
In many individuals, osteoarthritis occurs secondary to developmental abnormalities. Because osteoarthritis tends to occur well after maturity is reached, and is well established at the time it is recognized, successful treatment of this disease is extremely difficult Juvenile Osteochondritis Dissecans (JOCD) is one disease of developing individuals that results in osteoarthritis. In the present study, we demonstrate that, during normal skeletal maturation, areas of the femoral condyles that are predilection sites for JOCD have reduced vascularity. There is little prior literature exploring this possibility in humans, due to the scarcity of appropriate cadaveric specimens and to the lack methodology to examine vasculature in these sites. We hypothesize that the unique utilization of tissue inherent MRI contrast 1,2,3 will allow determination of vascular supply to growth cartilage and will offer new insights into predilection sites of knee JOCD.Methods
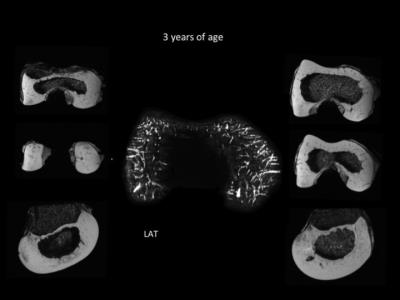
Twenty-two knees (11 female, 11 male) from pediatric cadavers, aged 1 month to 10 years, were provided by Allosource Inc, SA, USA. A waiver for IRB approval applied. 3-D gradient echo data suitable for susceptibility weighted imaging(SWI) or quantitative susceptibility mapping (QSM) were obtained from each intact specimen to visualize the vascular canals in epiphyseal cartilage of the distal femur. For best resolution, all specimens were imaged at ultra-high field MRI at 9.4 or 7 Tesla, depending on the size of the specimen. For proof of clinical feasibility, an SWI sequence was added to an MRI protocol of an 8-year-old boy under a current IRB protocol. For each distal femur, a bone-to-cartilage ratio was calculated. 3-D visualization of the vascular canals was achieved using QSM post-processing.Results
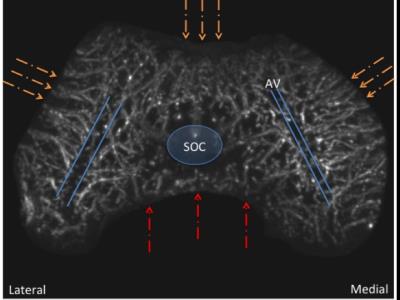
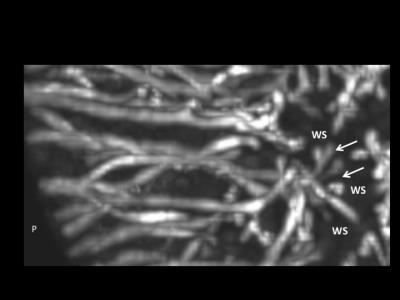
The femur contains two distinct sources of vascular supply: peripheral and central. The peripheral vascular bed supplies the medial and lateral condyles as well as the anterior trochlea. The central vascular bed supplies the intercondylar notch from posterior to anterior. The two vascular beds do not anastomose, but instead create an avascular watershed along the central aspect of each femoral condyle. The posterior intercondylar vascular bed regresses early during development. The central aspect of the medial femoral condyle and the lateral aspect of the trochlear ridge are the last sites to acquire their adult shape due to their larger volumes. A developmental mismatch that includes an asymmetrical articular epiphyseal cartilage complex (AECC) and symmetrical secondary ossification center (SOC) leaves these areas of remaining thick epiphyseal cartilage with progressively sparse vascular supply during the later stages of maturation.Discussion
This study has important clinical implications, since many of the leading causes of osteoarthritis are due to developmental abnormalities. Once osteoarthritis occurs, there is no cure. JOCD is one of the causes of premature osteoarthritis originating in developmental abnormalities during skeletal 4 This has been described previously in animal species 5,6,7, but not in In the present study we demonstrate that normal developmental stages of skeletal maturation may create areas of decreased vascular supply relative to tissue volume. Our most significant finding, that there are two distinct segmental vascular beds in the distal femoral epiphysis that lack anastomoses, has important implications for the viability of epiphyseal cartilage in predilection sites of JOCD. We observed that the central intercondylar vascular bed regresses much earlier than the peripheral lateral, anterior and medial vascular bed, leaving asymmetric thick areas of epiphyseal cartilage more distant from the remaining vascular supply. Importantly, these distant, more sparsely vascularized regions of the medial femoral condyle and lateral trochlea coincide with the predilection sites of JOCD.
Conclusion
Utilization of tissue inherent MRI contrast provided identification of cartilage canal vessels in epiphyseal cartilage. Importantly, this technique is feasible in pediatric patients and does not require administration of intravenous contrast. We demonstrated that, during normal maturation of the distal femur, areas of reduced vascularity occur. This is due, in part, to the fact that the distal femur contains two distinct sources of vascular supply, peripheral and central, that do not anastomose. The central vessels involute much earlier in development than the peripheral vessels, leaving thick areas of epiphyseal cartilage distant from the remaining peripheral vessels. Importantly, these distant and/or sparsely vascularized regions are the predilection sites of JOCD.Acknowledgements
No acknowledgement found.References
1. Nissi MJ, Toth F, Zhang J, et al. Susceptibility weighted imaging of cartilage canals in porcine epiphyseal growth cartilage ex vivo and in vivo. Magnetic resonance in medicine. 2014;71(6):2197-205.
2. Nissi MJ, Toth F, Wang L, Carlson CS, Ellermann JM. Improved Visualization of Cartilage Canals Using Quantitative Susceptibility Mapping. PloS one. 2015;10(7):e0132167
3. Toth F, Nissi MJ, Zhang J, et al. Histological confirmation and biological significance of cartilage canals demonstrated using high field MRI in swine at predilection sites of osteochondrosis. J Orthop Res. 2013;31(12):2006-12.
4. Nepple JJ, Milewski MD, Shea KG. Research in Osteochondritis Dissecans of the Knee: 2016 Update. J Knee Surg. 2016: 29(7):533-538. Epub 2016 Aug 17.
5. Carlson CS, Hilley HD, Henrikson CK, Meuten DJ. The ultrastructure of osteochondrosis of the articular-epiphyseal cartilage complex in growing swine. Calcif Tissue Int. 1989:38(1):44-51.
6. Olstad, K, Ekman S, Carlson CS. An update on the Pathogenesis of Osteochondrosis. Vet Pathol 2015:52(5):785-802.
Figures