0642
1D Partial Fourier Parallel MR imaging with deep convolutional neural networkShanshan Wang1, Ningbo Huang1,2, Tao Zhao1,3, Yong Yang2, Leslie Ying4, and Dong Liang1
1Paul C. Lauterbur Research Center for Biomedical Imaging, SIAT, Chinese Academy of Sciences, Shenzhen, People's Republic of China, 2School of Computer Science and Technology, Changchun University of Science and Technology, Changchun, People's Republic of China, 3College of Mining and Safety Engineering, Shandong University of Science and Technology, Qingdao, People's Republic of China, 4Department of Biomedical Engineering and Department of Electrical Engineering, The State University of New York, NY, United States
Synopsis
This paper develops a multi-coil SuperCNN network for 1D Partial Fourier Parallel MR imaging. With the utilization of enormous existing undersampled multi-channel images as inputs and their corresponding square root of sum-of-squares of images obtained from the fully sampled data as labels, the network is trained to identify the nonlinear mapping relationship and then performed as a predicator to reconstruct the online MR images. Experimental results on an in vivo dataset show that the proposed multi-coil SuperCNN is able to reconstruct more accurate MR images in less time compared to GRAPPA and SPIRiT from the same amount of undersampled data.
Introduction
Parallel imaging has been routinely used to accelerate imaging in MRI scanners and therefore accurate multi-coil undersampled MR image reconstruction is of great importance. Besides implicitly or explicitly exploring spatial sensitivity of multiple coils in conjunction with gradient encoding1,2, researchers have also devoted endless endeavors to incorporating prior information for accurate parallel MR reconstruction, such as sparsity3, low-rank4, multilayer perceptron5 and so on. Based on the observation that the multi-channel MR images have redundancy and convolutional neural network (CNN) possess strong capability in automatic feature extraction, correlation exploration and nonlinear relationship description6,7, we design a multi-coil SuperCNN to explore the local correlation in multi-channel images and draw valuable prior from the available big MR datasets for accurate multi-coil MR image reconstruction. Then the trained network is used to predict online image from the undersampled multi-channel data.Theory and method
The proposed work consists of two main parts: offline training and online imaging. For the offline training, there are two major ingredients namely the network design and the processing of big datasets for training samples. Fig.1 presents a general description of the forward process for the multi-coil superCNN network with one sample and the overall training framework. As can be seen from the figure, we extract overlapping image patches from the undersampled multi-coil images and use them as the inputs for the network. While for the output, the label is chosen as the corresponding patch from the square root of the sum-of-squares (SOS) of fully sampled images. With enormous high quality data included, the big data assist the network training. Once the network trained, we utilize it to obtain high quality reconstructions from the multi-coil undersampled input images.Experiment
We collected 2D fully sampled multi-slices data obtained by the 3T scanner (SIEMENS MAGNETOM Trio) in our institute from over 100 volunteers with a 12 channel head coil. Informed consent was obtained from the imaging subject in compliance with the Institutional Review Board policy. Undersampled measurements were retrospectively obtained using Hamming filtered asymmetrical 1D partial Fourier mask which has been approved optimal in our another abstract. As shown in Fig. 2, we use three layers of convolution for the network with the following configurations (64 nodes for the 1st layer with a kernel size 9*9, 32 nodes for the 2nd layer with a kernel size of 5*5, 1 node with size of 5*5). The multi-coil SuperCNN offline training took almost three days on a workstation equipped with 128G memory and a processor of 16 cores (Intel Xeon (R) CPU E5-2680 V3 @2.5GHz). Then the trained network was evaluated on in-vivo sagittal brain dataset which were acquired on a 3T scanner (SIEMENS MAGNETOM Trio) with a 12-channel head coil by T2-weighted turbo spin-echo (TSE) sequence (TE=76.0ms, TR=5090ms, FOV=18×18 cm, matrix=256×270, slice thickness=2.9mm).Result and discussion
Fig. 2 presents the test results of SPIRiT, GRAPPA and the proposed method from 25% of sampled data. The ground truth (a) and the undersampling trajectory (b) for SPIRiT and GRPPA are also provided. Our undersampling trajectory is a Hamming filtered asymmetrical 1D partial Fourier scheme (c). As can be seen from the test results, there are obvious noise in SPIRiT and GRAPPA. For a closer look, we have enlarged the white box enclosed parts for comparisons which show that GRAPPA and SPRiT also suffer from some aliasing artifacts. On the other hand, our method has provided a clearer image closer to the ground-truth.Conclusion
This work designs and trains a multi-coil SuperCNN for 1D partial parallel MR imaging. The network absorbs prior information from a huge number of existing high-quality fully-sampled multichannel data and then serves as a predicator to restore lost information from the undersampled multi-channel data. The experimental results have shown that the proposed method has produced an image with less noise and artifacts compared to the classical GRAPPA and SPIRiT methods.Acknowledgements
Grant support: China NSFC 61471350, 61601450, the Natural Science Foundation of Guangdong 2015A020214019, 2015A030310314, the Basic Research Program of Shenzhen JCYJ20140610152828678, JCYJ20160531183834938, JCYJ20140610151856736 and the youth innovation project of SIAT under 201403 and US NIH R21EB020861 for Ying.References
1. Pruessmann KP, Weiger M, Scheidegger MB, and Boesiger P. SENSE: Sensitivity encoding for fast MRI. MRM, 1999; 42(5)952-962. 2. Mark G, Peter M. J, Robin M. H, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). MRM, 2002; 47(6):1202-1210. 3. Michael L, David D and John M. P. Sparse MRI: The application of compressed sensing for rapid MR imaging. MRM, 2007; 58(6):1182-95. 4. Justin P. H and Jingwei Z. P-LORAKS: LOW-rank modeling of local k-space neighborhoods with parallel imaging data. MRM, 2016; 58(6):1499-1514. 5.Kwon K, Kim D, Seo H, Cho J, Kim B and Park H. Learning-based reconstruction using artificial neural network for higher acceleration. ISMRM, 2016; 1801 6. Shan W, Zhenghang S, Leslie Y, Xi P, Shun Z, et al. Accelerating magnetic resonance imaging via deep learning. IEEE ISBI, 2016; 514-517. 7. Shan W, Zhenghang S, Xi P and Dong L. Exploiting deep convolution neural network for fast resonance imaging. ISMRM, 2016; 1778.Figures
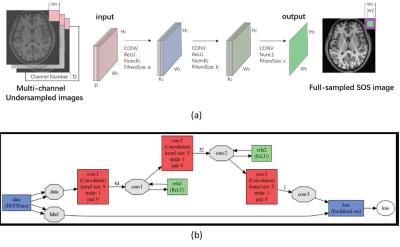
Figure. 1:
Fig. 1 From top to bottom: (a) A general
description of the forward process of the offline SuperCNN network with one training pair and (b) the overall offline training framework.
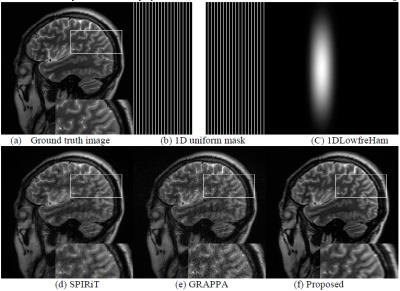
Figure 2: From left to right and top to bottom:
Ground-truth MR image, 1D uniform undersampling mask for SPIRiT and GRAPPA,
Hamming filtered asymmetrical 1D partial Fourier sampling trajectory, the
reconstructions of the SPIRiT, GRAPPA and the proposed method from 25% of
sampled data.