0639
Dark-Blood Late Gadolinium Enhanced MRI: A Novel Method without Additional Magnetization Preparation for Improved Myocardial Scar Detection1Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 2Philips, Guildford, United Kingdom
Synopsis
Late gadolinium enhanced (LGE) MRI often suffers from poor scar-to-blood contrast when used for detection of endocardial scar due to the bright signal of adjacent blood. We report a method that significantly reduces left ventricular blood signal by setting a shorter inversion time in combination with a phase-sensitive inversion recovery (PSIR) sequence. Nulling the left ventricular blood signal with PSIR significantly increases scar-to-blood contrast since blood signal and scar signal no longer have similar signal levels. As no additional magnetization preparation is used, clinical application on current MR systems is readily available without the need for software modifications or additional training.
Purpose
Late gadolinium enhancement (LGE) has become the gold standard in the assessment of myocardial viability, as LGE is able to excellently depict myocardial infarction (MI) and macroscopic scarring from viable myocardium[1]. However, due to the bright signal of adjacent blood within the left ventricle (LV), the apparent volume of the damaged myocardial tissue can be significantly reduced, or even obscured. Obscuration particularly occurs in cases of thin subendocardial scarring, mostly caused by coronary artery disease and subsequent MI. In order to allow improved expert confidence in assessing the presence of scar and its transmurality, efforts have been made to reduce blood signal using various additional magnetization preparation schemes[2-5]. We report a method that significantly reduces LV blood signal in LGE, without using additional magnetization preparation, which is readily available on current MR systems.
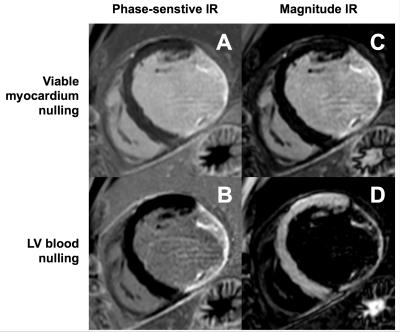
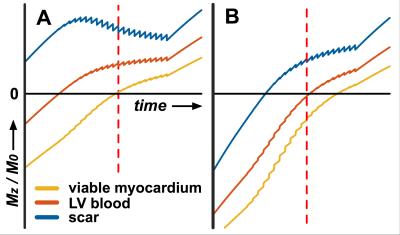
Phase-sensitive inversion recovery (PSIR) is commonly used for LGE image acquisition as it avoids the need for precise selection of the inversion delay time (TI) to null viable myocardium[6]. PSIR also shows the recovered longitudinal magnetization (Mz) differently in the corrected real image produced for clinical assessment: -Mz is darkest, nulled tissue is mid-gray, and +Mz is bright; whereas in a magnitude image nulled tissue is darkest and both -Mz and +Mz are bright. PSIR is used routinely with nulled viable myocardial tissue, and the clinical observer may adjust window levels to further darken viable myocardial tissue, mimicking a magnitude image representation. However, the PSIR gray scale range provides an opportunity to achieve a darker blood signal whilst preserving bright scar signal by setting a shorter TI such that the LV blood signal is close to the null point of recovery.
Methods
Imaging was performed on nine male patients (63±11y) with ischaemic myocardial scar at 1.5T (Ingenia; Philips, The Netherlands) 15-33 minutes after intravenous injection of 0.2 mmol/kg gadobutrol (Gadovist; Bayer, Germany). A Look-Locker sequence was performed first to identify the two TIs at which nulling of the viable myocardium and LV blood signal occurs. Subsequently, single slice breath-hold LGE images were acquired at those two TIs, in a randomized order, using a PSIR turbo field echo pulse sequence (TE/TR 3.0/6.1 ms, flip angle 25° (PSIR 5°), FOV 350x350 mm, slice thickness 10 mm, acquisition matrix 220x170, reconstructed voxel size 0.91x0.91 mm2, 19 lines acquired every other RR-interval) during the mid-diastolic resting period. A dedicated noise scan without any RF excitations was performed to measure the noise level. The applied scaling in the stored DICOM data was removed by converting the data to floating point values as this reflects the true MR signal range directly after reconstruction. Regions of interest were drawn in the viable myocardium, infarcted myocardium (scar), and the LV blood pool in each image by an expert observer (>10y cardiovascular MRI experience) who was blinded to image type. Signal-to-noise ratios (SNRs) and contrast-to-noise ratios (CNRs) were calculated. CNRs were statistically compared using a paired sample t-test and visualized using a Bland-Altman bias plot. A Shapiro-Wilk test was performed to confirm normality of data. Bloch simulations were performed taking into account all sequence details such as start-up echoes, reference acquisitions, and flip angle sweeps.Results
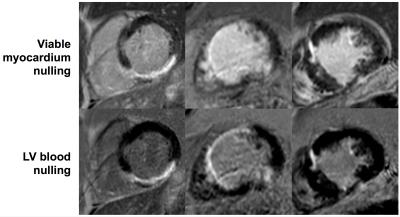
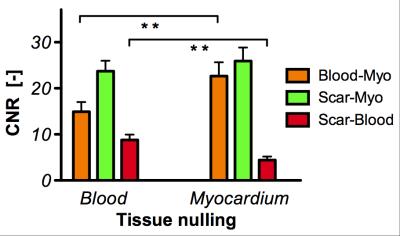
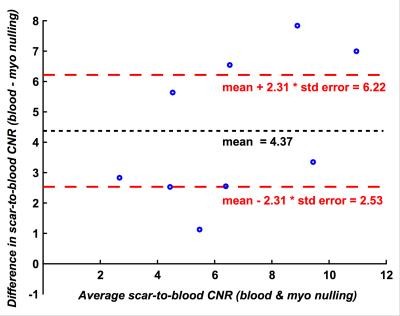
Imaging at both inversion times (LV blood nulling and viable myocardium nulling) was performed successfully in all patients at 22±4.6 min and 23±6.2 min post-injection, respectively (fig. 1 & 2). The inversion time for LV blood nulling and viable myocardium nulling was 168±28 ms and 259±25 ms, respectively. The scar-to-blood contrast in the PSIR images was significantly (p<0.001) increased when nulling blood instead of viable myocardium while a change in scar-to-myocardium contrast was not detected (p=0.15) (fig. 3). Bland-Altman analysis confirmed these findings by demonstrating a significant bias of +4.37 (95% CI [+2.53, +6.22], fig. 4) in scar-to-blood CNR, which is a 99% increase when nulling LV blood. The Shapiro-Wilk test confirmed normality of data. Bloch simulations confirmed the nulling of viable myocardium and LV blood when using corresponding sequence and tissue parameters (fig. 5).Discussion
Nulling viable myocardium for PSIR LGE is a continuation of routine clinical practice from the time non-phase-sensitive (magnitude) images were used for LGE. Nulling the LV blood signal instead for PSIR significantly increases scar-to-blood contrast since blood signal and scar signal no longer have similar signal levels. We introduced a novel method that allows visualization of contrast-enhanced tissues while suppressing the blood pool, thereby improving subendocardial scar conspicuity in PSIR LGE. As no additional magnetization preparation is used, clinical application on current MR systems is readily available without the need for software modifications or additional training.Acknowledgements
References
[1] Kellman et al. Cardiac Imaging Techniques for Physicians: Late Enhancement. JMRI. 2012;36:529-542
[2] Peel et al. Dual Inversion-Recovery MR Imaging Sequence for Reduced Blood Signal on Late Gadolinium-enhanced Images of Myocardial Scar. Radiology. 2012;264:242–249
[3] Liu et al. Improved Delayed Enhanced Myocardial Imaging With T2-Prep Inversion Recovery Magnetization Preparation. JMRI. 2008;28:1280-1286
[4] Basha et al. Black Blood Late Gadolinium Enhanced (BB-LGE) using a joing T2 Magnetization Preparation and Inversion Preparation. Proc Intl Soc Magn Reson Med. 2015;23:0184
[5] Kim et al. Black-Blood Contrast-Enhanced MRI: Validation of a Novel Technique for the Diagnosis of Myocardial Infarction. Proc Intl Soc Magn Reson Med. 2015;23:0662
[6] Kellman et al. Phase-Sensitive Inversion Recovery for Detecting Myocardial Infarction Using Gadolinium-Delayed Hyperenhancement. MRM. 2002;47:372-383
Figures