0630
Hyperpolarized [1,4-13C2]Fumarate is a probe of necrosis in myocardial infarction1Department of Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom, 2Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, Oxford, United Kingdom, 3Department of Physics, University of Oxford, Oxford, United Kingdom, 4Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 5Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 6MR Centret, Århus University Hospital, Skejby, Denmark
Synopsis
Previous work has shown that hyperpolarized [1,4-13C2]fumarate is a probe of cellular necrosis. We demonstrate here that the ratio of cardiac hyperpolarized malate to fumarate is increased by a factor of $$$\sim$$$82 one day after cryoinduced myocardial infarction in rats, decreasing to an $$$\sim$$$30-fold increase one week after injury. We additionally image this injury with a novel spiral multiband pulse sequence. Hyperpolarized fumarate therefore forms a sensitive probe of myocardial injury in vivo, and could form a clinical monitor of cellular damage and necrosis after infarction.
Purpose
Myocardial infarction (MI) is characterized by cellular necrosis and the subsequent cascade of structural and functional adaptations to it. The accurate and reproducible quantification of the region of necrosis immediately following myocardial infarction has been identified as a clinical requirement, and techniques such as late gadolinium imaging or T2 weighted imaging aim to infer the region of necrosis indirectly.[1] At present, there is no clinically validated mechanism for detecting necrosis directly in vivo. The injection of hyperpolarized [1,4-13C2]fumarate and its subsequent conversion to [1,4-13C2]malate has probed cellular necrosis in vivo in tumours[2] and following acute kidney injury.[3] We therefore investigated the use of hyperpolarized [1,4-13C2]fumarate in a rodent model of myocardial infarction.Methods
Hyperpolarization: [1,4-13C2]fumaric acid ($$$3.23\,\text{mmol}$$$; Cambridge Isotopes) was dissolved in $$$8.74\,\text{mmol}$$$ of DMSO containing $$$11.48\,\mu\text{mol}$$$ of a trityl radical (AH111501; GE Healthcare) and $$$0.48\,\mu\text{mol}$$$ of a gadolinium chelate (Dotarem, Guerbet). A $$$40\,\text{mg}$$$ aliquot was polarized per experiment (prototype hyperpolarizer, $$$45\,\text{min}$$$ at $$$94\,\text{GHz}$$$) before dissolution (in $$$6\,\text{ml}$$$ NaOH, final concentration $$$20\,\text{mM}$$$). Infusion ($$$2\,\text{ml}$$$) was via a tail vein cannula over $$$20\,\text{s}$$$ following dissolution
Myocardial Infarction: Six female Wistar rats ($$$\sim200\,\text{g}$$$, Harlan) were divided into two groups (MI or control, $$$n=3$$$), and subject to cryoinduced myocardial infarction.[4] Control animals were subject only to pericardial removal. Pre-operative and postoperative analgesia was provided. All experiments were performed with appropriate ethical review.
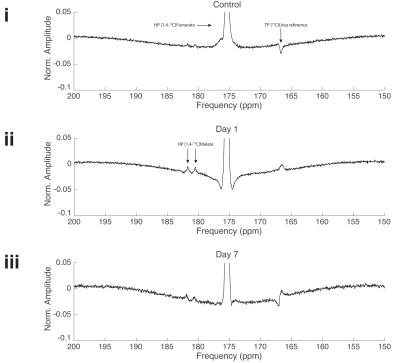
Spectroscopy: An actively detuned transmit/surface receive coil was used (72 mm 1H/13C proton/carbon birdcage transmit with $$$40\,\text{mm}$$$ two-channel 13C surface receive; Rapid Biomedical GMBH, Rimpar, Germany) as described previously.[5] A $$$5\,\text{M}$$$ 13C-urea phantom was included as a per-animal FA and frequency reference. Slice-selective spectra ($$$200\,\mu\text{s}$$$ gauss, $$$20\,\text{mm}$$$ thick, TR$$$=\sim1\,\text{s}$$$, bandwidth $$$10\,\text{kHz}$$$, FA $$$10^\circ$$$) were acquired from the heart in end-systole. Cardiac function was assessed by CINE.[6] Spectra were summed for $$$60\,\text{s}$$$ following the appearance of the [1,4-13C2]fumarate peak, and quantified with AMARES, with the reported malate:fumarate ratio being that of total visible malate to fumarate.
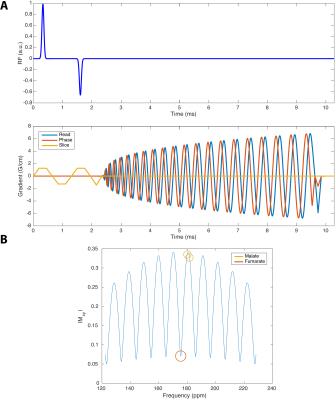
Multiband Imaging: A hybrid multiband spatial-spectral RF excitation pulse[7] was designed to simultaneously excite [1,4-13C2]fumarate at a $$$4^\circ$$$ FA, and both [1,4-13C2]malate resonances at $$$\sim20^\circ$$$, and a slab thickness of $$$20\,\text{mm}$$$. A multi-echo spiral readout[8] was constructed to maximise the effective number of signal averages over all chemical species in the subsequent reconstruction, with FID acquisition every seventh echo ($$$\text{TE}=2.1,\,3.8,\,5.5,\,7.2,\,8.9,\,10.6,\,12.3$$$ FID $$$2.1\,\text{ms}$$$). The spiral trajectory was designed with a nominal FOV of $$$80\times80\,\text{mm}^2$$$, readout bandwidth of $$$250\,\text{kHz}$$$, TR$$$=1\,\text{RR}$$$ interval, nominal acquisition in-plane resolution $$$2\times2\,\text{mm}^2$$$. The pulse sequence is shown in Fig. 1. The k-space trajectory was predicted using a premeasured gradient impulse response function.[9] The multiecho reconstruction was performed prior to NUFFT[10] ($$$30\,\text{Hz}$$$ Lorentzian filtered; reconstructed resolution $$$5.62\times5.62\,\text{mm}^2$$$; nominal $$$128\times128$$$ matrix). $$$n=1$$$ control and infarcted animals were scanned; other details as above.
Results
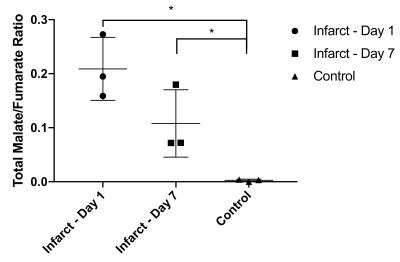
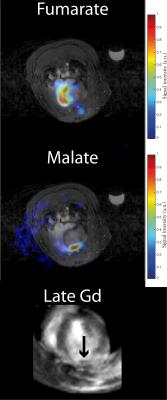
Myocardial infarction was confirmed via CINE. The [1,4-13C2]malate:[1,4-13C2]fumarate ratio visible in the in vivo infarcted heart was $$$0.21\pm0.03$$$ one day post infarction, dropping to $$$0.077\pm0.005$$$ seven days post infarction compared to $$$0.0025\pm0.001$$$ in controls, corresponding to an $$$\sim82$$$-fold increase in visible malate signal post-infarction (Figs. 2&3; Cohen's $$$d=5.01$$$). Very little [1,4-13C2]malate was observed in control animals, potentially indicating the negligible rate of transport of the probe within the lifetime of the hyperpolarized experiment. As shown in Fig. 4, it was possible to resolve fumarate perfusing the heart, and malate in the region of the infarction in the basal LV in the infarcted animal shown; production was not visible in controls. This region of necrosis was smaller than the corresponding wall motion abnormality in CINE, and smaller than the detected region of enhancement by late gadolinium.Discussion
Malate production in the infarcted heart appears promising as a sensitive in vivo probe of necrosis following MI. We have shown that the technique could offer the first non-invasive measurement of necrotic cell death in vivo. The region of malate signal detected in the heart following MI appears distinct from the region of late gadolinium enhancement, which is consistent with gadolinium revealing aberrant vasculature and regions of fibrosis rather than necrosis directly. Hyperpolarized [1,4-13C2]fumarate is therefore suited to studying the decline into heart failure following MI. In contrast to the use of blood-borne biomarkers such as troponin to detect necrosis, hyperpolarized [1,4-13C2]fumarate can be spatially localised, and the rapid timescale of hyperpolarized experiments is uniquely suited to acute MR investigations.Conclusions and Future Work
Hyperpolarized [1,4-13C2]fumarate can be used to specifically identify cardiomyocyte necrosis both acutely following myocardial infarction and up to one week later. Future work will investigate its co-localization to the region of myocardial injury, and investigate the specificity and sensitivity of the technique in population studies compared to troponin.Acknowledgements
The authors would like thank financial support from an EPSRC Doctoral Training Centre Grant and Doctoral Prize Fellowship (refs. EP/J013250/1 and EP/M508111/1) and St. Hugh's College, Oxford. We also acknowledge financial support from the British Heart Foundation (Fellowships FS/10/002/28078 & FS/11/50/29038, Programme Grant RG/11/9/28921).
References
[1] P. G. Masci, J. Bogaert, Cardiovasc. Diagn. Ther. 2012, 2, 113–27.
[2] F. A. Gallagher, M. I. Kettunen, D.-E. Hu, P. R. Jensen, R. I. ’. Zandt, M. Karlsson, A. Gisselsson, S. K. Nelson, T. H. Witney, S. E. Bohndiek, G. Hansson, T. Peitersen, M. H. Lerche, K. M. Brindle, Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 19801–19806.
[3] M. R. Clatworthy, M. I. Kettunen, D.-E. Hu, R. J. Mathews, T. H. Witney, B. W. C. Kennedy, S. E. Bohndiek, F. A. Gallagher, L. B. Jarvis, K. G. C. Smith, K. M. Brindle, Proc. Natl. Acad. Sci. U. S. A. Aug. 2012, 109, 13374–13379.
[4] E. J. van den Bos, B. M. Mees, M. C. de Waard, R. de Crom, D. J. Duncker, Am J Physiol Hear. Circ Physiol 2005, 289, H1291–300.
[5] J. J. Miller, A. Z. Lau, I. Teh, J. E. Schneider, P. Kinchesh, S. Smart, V. Ball, N. R. Sibson, D. J. Tyler, Magn. Reson. Med. May 2015, 75, 1515–1524.
[6] J. E. Schneider, P. J. Cassidy, C. Lygate, D. J. Tyler, F. Wiesmann, S. M. Grieve, K. Hulbert, K. Clarke, S. Neubauer, J. Magn. Reson. Imaging Dec. 2003, 18, 691–701.
[7] A. Sigfridsson, K. Weiss, L. Wissmann, J. Busch, M. Krajewski, M. Batel, G. Batsios, M. Ernst, S. Kozerke, Magn. Reson. Med. May 2014, DOI 10.1002/mrm.25294.
[8] F. Wiesinger, E. Weidl, M. I. Menzel, M. A. Janich, O. Khegai, S. J. Glaser, A. Haase, M. Schwaiger, R. F. Schulte, Magn. Reson. Med. July 2012, 68, 8–16.
[9] J. H. Duyn, Y. Yang, J. A. Frank, J. W. van der Veen, J. Magn. Reson. May 1998, 132, 150–3.
[10] J. Fessler, B. Sutton, IEEE Trans. Signal Process. Feb. 2003, 51, 560–574.
Figures