0629
Optimization of mpMRI protocol in differentiating muscle-invasive from non-muscle invasive Bladder Cancer: is there room for Diffusion Tensor Imaging (DTI)?1Department of Radiological Sciences, Sapienza University of Rome, Rome, Italy
Synopsis
Staging of BCa is critical, especially in differentiating non-invasive from muscle infiltrative lesions, as patient management strongly differs according to stage. Currently, patients with bladder lesions undergo two invasive procedures to diagnose and eventually treat the tumor if it is superficial. In this study we showed that mp-MRI has high capability for differentiating superficial from deep lesions, thanks also to DTI ability to directly visualize detrusor muscle layers. Mp-MRI could therefore be included systematically in the diagnostic evaluation of patients with suspicious bladder lesions in order to possibly avoid the first diagnostic cystoscopy once detrusor muscle invasion has been excluded.
Introduction
Bladder cancer (BCa) is a common cancer worldwide and one of the most expensive to manage1,2. This tumor is best classified as either non-muscle invasive (NMIBC) or muscle-invasive (MIBC) as this reflects biology and alters treatment intent3. Up to one third of invasive BCa are initially staged as NMIBC at transurethral resection4 . Given the limitations of current clinical staging approaches, improved radiological tools are needed. The aim of this study is to examine the accuracy of multiparametric magnetic resonance imaging (mp-MRI) in differentiating NMIBC from MIBC. Morphological and functional sequences were tested, including perfusion weighted imaging (PWI), diffusion weighted imaging (DWI) and diffusion tensor imaging (DTI), with the fractional anisotropy (FA) methodology.Methods
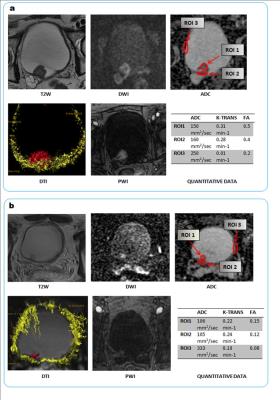
Between January and June 2016, patients with suspected or confirmed bladder lesions were enrolled. Before bladder resection, they underwent an MRI study which included: turbo spin-echo T2-weighted images in axial and sagittal planes; DW images obtained during free-breathing in the axial plane by using a single-shot spin-echo echoplanar sequence with chemical shift-selective fat-suppression techniques; PWI images acquired with 25 acquisitions of 4-5 seconds per acquisition, 20-150 seconds after intravenous administration of contrast medium; T1-weighted images post contrast medium injection with a fat-suppressed 3D volumetric spoiled gradient-echo sequence (aimed at complete pelvic examination) and DTI data acquisition. Images were independently analyzed in two reading sessions by two Radiologists, with 10 and 3 years of urogenital experience, respectively. Four image sets (T2-weighted plus PWI, T2-weighted plus DWI, T2-weighted plus DWI plus PWI and T2-weighted plus DWI plus PWI plus DTI) for each patient were interpreted qualitatively without knowledge of pathology findings. During the first session, each Radiologist interpreted T2-weighted plus PWI images according to criteria already described in literature5,6 without formulating a definitive diagnosis of muscular invasion. In the second session, the remaining images were analyzed, including DWI sequences and DTI imaging results, which were assessed both qualitatively and a quantitatively. Quantitative analysis was based on the results of the third image set (T2-weighted + PWI + DWI), used to formulate the definitive report. All cases considered at least as “slightly suspicious” (i.e. all cases with a score ≥ 3) after reading the aforementioned image set (22 lesions) were analyzed separately from the others (39 lesions), fig.1. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy in detecting muscle invasion were calculated for all image sets, and receiver operating characteristic (ROC) curves were generated.Results
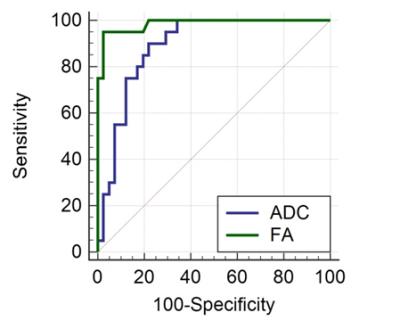
In total, 61 patients entered the study. We resected 69 tumors of which 45 were solitary and 10 multifocal. Tumors were staged as pTa-1 (n=41), pT2 (n=20), pT3 (n=3) and pT4 (n=5). We excluded the 8 pT3-4 lesions, leaving a final total of 61 tumors for analysis. The greatest accuracy for invasion was seen with all MR sequences (T2-weighted, PWI, DWI and DTI: AUROC 0.99), compared to only T2W with PWI (AUROC 0.73), fig. 2. Quantitative analysis showed that both ADC and FA values were significantly different in NMIBC and MIBC when calculated at the interface between tumor and detrusor muscle. ROC analysis suggested FA was significantly superior to ADC (AUC for FA = 0.985 [95% CI 0.914-1.000] vs. ADC = 0.889 [95% CI 0.782-0.955]), fig. 3.Discussion
Overall, we found that combined protocols offered greatest accuracy and that these outperformed T2-weighted plus PWI sequences, which have been show to match MDCT previously7, our data suggest contemporary mpMRI should outperform CT for tumor staging. We found only two cases that were misclassified by the MRI exam. Of these, one T2 lesion had early microscopic invasion at histopathology (T2a) and was classified as NMIBC, whilst one pT1 lesion showing a very similar signal intensity of tumor and sub-mucosa at PWI was labeled as MIBC. The innovation of the multiparametric evaluation in this study is the introduction of DTI imaging. In cases where DWI and ADC sequences were inconclusive and an interpretative doubt about bladder invasion was posed, we resorted to DTI as decisive tool, because it allows direct visualization of bladder wall muscle layers. The inclusion of DTI into an mp-MRI protocol allowed us to reach a diagnostic accuracy of 95% in differentiating MIBC from NMIBC.Conclusion
Since mpMRI, including DTI, has high accuracy in determining detrusor muscle invasion, we propose it could be included in the diagnostic evaluation of patients suspected of having Bca. If mpMRI suggests muscle invasion, one wonders whether in the future the first TURB patients undergo in order to confirm the diagnosis could be excluded. This could theoretically expedite radical treatment and allow the urologist to avoid unnecessary invasive procedures.Acknowledgements
No acknowledgement found.References
1. Chavan S, Bray F, Lortet-Tieulent J, Goodman M, Jemal A. International variations in bladder cancer incidence and mortality. Eur Urol. 2014 Jul;66(1):59–73.
2. Svatek RS, Hollenbeck BK, Holmang S, Lee R, Kim SP, Stenzl A, Lotan Y. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014 Aug;66(2):253–62.
3. Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, Hernandez V, Kaasinen E, Palou J, Roupret M, van Rhijn BWG, Shariat SF, Soukup V, Sylvester RJ, Zigeuner R. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2016 Jun.
4. Kulkarni GS, Hakenberg OW, Gschwend JE, Thalmann G, Kassouf W, Kamat A, Zlotta A. An updated critical analysis of the treatment strategy for newly diagnosed high-grade T1 (previously T1G3) bladder cancer. Eur Urol. 2010 Jan;57(1):60–70.
5. Hayashi N, Tochigi H, Shiraishi T, Takeda K, Kawamura J. A new staging criterion for bladder carcinoma using gadolinium-enhanced magnetic resonance imaging with an endorectal surface coil: a comparison with ultrasonography. BJU Int. 2000 Jan;85(1):32–6.
6. Tekes A, Kamel I, Imam K, Szarf G, Schoenberg M, Nasir K, Thompson R, Bluemke D. Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR Am J Roentgenol. 2005 Jan;184(1):121–7.
7. de Haas RJ, Steyvers MJ, Futterer JJ. Multiparametric MRI of the bladder: ready for clinical routine? AJR Am J Roentgenol. 2014 Jun;202(6):1187–95.
Figures