0612
Pharmacological arterial spin labeling reveals distinct mesocorticolimbic blood flow in Parkinson’s disease patients with compulsive reward-driven behaviors1Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Neurology, Vanderbilt University Medical Center, 3Radiology, Vanderbilt University Medical Center, 4Radiology, University of Alabama at Birmingham, 5Psychology, Vanderbilt University Medical Center
Synopsis
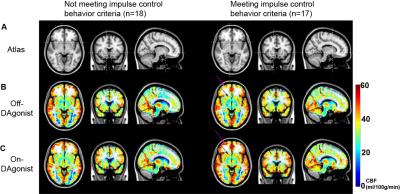
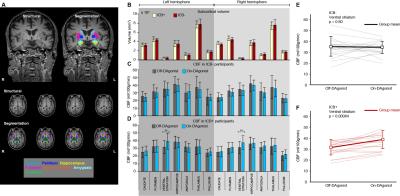
The overall goal of this work is to apply pharmacological arterial spin labeling (ASL) to investigate fundamental hypotheses regarding the role of dopamine agonist (DAgonist) therapy in patients with Parkinson’s disease and impulse control behavior (ICB). Parkinson’s disease patients (n=35; age range=40-79 years; gender=23/12 males/females) receiving DAgonist therapy, with (n=17) and without (n=18) DAgonist-induced ICB were scanned at 3T using cerebral blood flow (CBF)-weighted pCASL MRI. Region-of-interest analyses revealed significantly increased bilateral ventral striatal (P<0.01) CBF in patients with ICB in the On- DAgonist state; voxel-wise analysis of CBF confirmed widespread DAgonist-induced CBF increases in mesolimbic, mesocortical, and midbrain regions.
Introduction
The overall goal of this work is to apply pharmacological arterial spin labeling (ASL) to investigate fundamental hypotheses regarding the role of dopamine agonist (DAgonist) therapy in patients with Parkinson’s disease and impulse control behavior (ICB). Impulsive behaviors are a well-known side effect of DAgonist therapy for motor symptoms in Parkinson’s disease. Non-ergot agonists have an increased affinity to D2-like receptors (i.e., D3 and D2 receptors), and are causally linked to maladaptive behaviors commonly referred to as Impulse Control Disorder1. These behavioral changes are characterized by compulsive participation in reward-driven activities, implicating an aberrant effect of this class of medication on the mesocorticolimbic network. Previous imaging data suggest structural and functional differences localize to this network2, but a lack of quantitative investigations of physiology has resulted in little evidence of mechanisms. Here, we apply multi-modal structural and hemodynamic MRI before and after DAgonist therapy in patients in a novel application of pharmacological ASL.Methods
Parkinson’s disease patients (n=35; age range=40-79 years; gender=23/12 males/females) receiving DAgonist therapy, with (n=17) and without (n=18) DAgonist-induced impulsive and compulsive behaviors provided informed consent and were scanned at 3T (Philips Achieva). Behavioral symptom severity was quantified using the Questionnaire for Impulsive Compulsive Disorders in Parkinson’s Disease Rating Scale. Standard structural imaging including T1- and T2-weighted imaging, and angiography, was performed for white matter disease, volumetric, and vasculopathy assessment, respectively. To quantify medication effects on cerebral blood flow (CBF; ml/100g/min), pseudo-continuous ASL (pCASL)3 in the On- dopamine agonist and Off- dopaminergic state was applied. pCASL data were pair-wise subtracted and corrected for motion and partial volume effects. Quantitative CBF was compared pre- and post-DAgonist in pre-defined subcortical structures hypothesized to be involved in ICD in native space, and also co-registered to a standard atlas (MNI; 2mm) for a an exploratory whole-brain voxel-wise analysis. Corrected p-value<0.05 was required for significance.Results
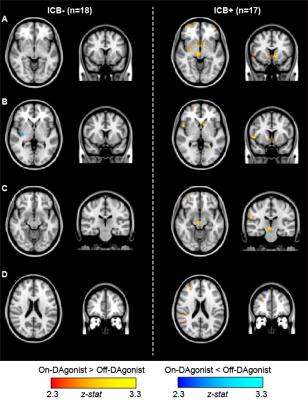
Region-of-interest analyses revealed significantly increased bilateral ventral striatal (P<0.01) CBF in Parkinson’s disease patients with impulsive and compulsive behaviors in the On- DAgonist state (Figs. 1 and 2). No differences were detected between groups in the Off- medication state or in subcortical volumes. When a broader striatal region was considered collectively (ventral striatum, caudate, and putamen), an increase in right striatal CBF was evident in patients with impulsive and compulsive behaviors (P=0.018) but not in those without (P=0.52). DAgonist-induced change in CBF in the ventral striatum correlated with the severity of these behaviors (Spearman’s rho=0.37, P=0.029). Voxel-wise analysis of CBF (Fig. 3) confirmed widespread DAgonist-induced CBF increases in mesolimbic, mesocortical, and midbrain regions.Discussion
Pharmacological ASL, which allows for quantitative metrics of brain function to be compared noninvasively between time points, was applied in patients with impulsivity pre- and post-DAgonist. These findings support the hypothesis that DAgonist specifically augments mesocorticolimbic and striato-nigro-striatal networks in Parkinson’s patients with impulsive compulsive behaviors. The involvement of key mesocortical (ventromedial prefrontal cortex) and mesolimbic (ventral striatum) regions in impulsive-compulsive drug response supports a wider literature linking addiction and other compulsive reward-driven behaviors to neurobiological changes in this network. The greater increases in the right hemisphere (Fig. 3) suggest a possible asymmetric regulation of reward-driven and compulsive behavior.Conclusion
This work extends previous imaging studies in ICB by linking clinical severity to acute DAgonist administration and concerted quantitative hemodynamic changes in key mesocorticolimbic structures.Acknowledgements
No acknowledgement found.References
1. Weintraub D, David AS, Evans AH, et al. Clinical spectrum of impulse control disorders in Parkinson's disease. Mov Disord. 2015;30(2):121-7.
2. Rao H, Mamikonyan E, Detre JA, et al. Decreased ventral striatal activity with impulse control disorders in Parkinson's disease. Mov Disord. 2010;25(11):1660-9.
3. Dai W, Garcia D, de Bazelaire C, et al. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic resonance in medicine. 2008;60(6):1488-97.
Figures