0608
Validation of axon diameter and density estimates by TractCaliber MRI in a biomimetic brain phantom1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 2Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 3Learning Research and Development Center, University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
We validate axon diameter and density estimates using a novel biomimetic brain phantom that emulates the microstructural features of white matter, consisting of hollow textile axons (“taxons”) on the micron scale with distinct intra- and extra-axonal compartments and crossing regions. Diffusion data acquired over a range of gradient strengths, directions and diffusion times were fitted with the TractCaliber approach. Taxon volume fractions were accurately estimated, and the diameters were slightly overestimated in areas with less densely packed fibers. Such phantoms may be useful for testing microstructural models of the diffusion signal, and for scanner and protocol calibration.
Purpose
Diffusion microstructural imaging techniques have attracted great interest in the last decade due to their ability to quantify axon diameter and packing density in white matter. The estimates of compartment size and packing density continue to be debated, in part due to the lack of a gold standard for validation and quality control. Anisotropic diffusion phantoms constructed out of solid or honeycombed polymer fibers have been useful for quantitative validation of diffusion MRI metrics (1, 2). The presence of hollow fibers with distinct intra- and extra-axonal compartments is essential for validating compartment-specific diffusion models. In this work, we use a recently developed biomimetic brain phantom with biologically meaningful diameters and crossing fibers to validate estimates of compartment size and packing density by TractCaliber MRI.Methods
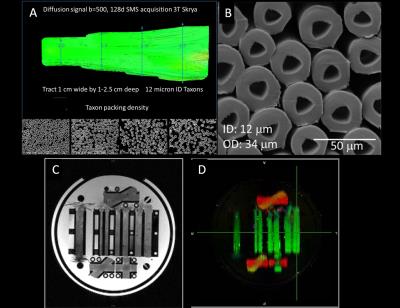
Phantom composition: The phantom used hollow multifilament polypropylene yarns produced by a melt-spinning extrusion technique to generate textile axons (“taxons”) with inner diameter of 12 µm and outer diameter of 34 µm (3) manufactured by Psychology Software Tools, Inc. (Pittsburgh, Pennsylvania). The taxons were arranged in parallel configuration within a 3D-printed modular phantom with a flat placeholder measuring 10 mm wide and of varying depth (10 to 25 mm) to achieve fiber packing densities of 40%, 50%, 60% and 80% (Fig. 1). Fiber crossings of 90º, 45º and 30º were created in separate areas of the phantom by interleaving the polypropylene filaments. The phantom was filled with distilled water by pressure filling.
Diffusion MRI experiments: All scans were performed on the dedicated high-gradient 3T CONNECTOM system with maximum gradient strength of 300 mT/m using a custom-made 64-channel head coil (4). A series of 2-mm isotropic resolution diffusion-weighted spin echo EPI images were acquired in the phantom using the following parameters: TR/TE = 4100/110 ms, diffusion gradient pulse duration δ = 8 ms, diffusion times Δ = 20, 26, 40, and 60 ms, and 256 non-collinear diffusion-encoding gradient directions with 20 interspersed b=0 images. The gradient strength was varied from 60 to 290 mT/m to produce b-values of 1000, 2000, 4000, 6000 and 9000 s/mm2 for each diffusion time. The total acquisition time was 9.5 hours.
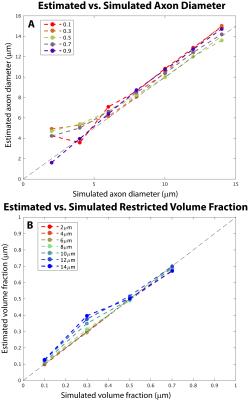
Simulations: Synthetic MRI data with infinite SNR was generated using the Monte Carlo diffusion simulator of Camino for diffusion within impermeable, hexagonal-packed cylinders with a range of diameters (2-20μm) and intra-axonal volume fractions (0.1-0.7) using the experimental parameters described above.
Data analysis: The experimental data was preprocessed to correct for distortions due to gradient nonlinearity, susceptibility effects, B0 drift, and eddy currents (5, 6). Axon diameter and volume fractions were obtained by the TractCaliber method (7). Generalized q-sampling imaging was used to determine the principal fiber direction in each voxel. Spherical harmonics expansion with Laplace-Beltrami regularization was used to interpolate the data to determine the average signal perpendicular to the principal fiber direction. A three-compartment model of intra-axonal, extra-axonal, and free diffusion was fitted to the data to obtain estimates of axon diameter, restricted and free diffusion volume fractions, and hindered diffusivity through Markov chain Monte Carlo sampling.
Results
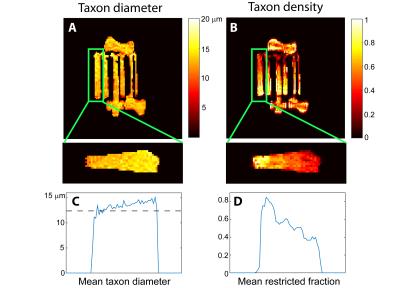
In simulations, the TractCaliber method recovered estimates of axon diameter and intra-axonal volume fraction consistent with simulated diameters >5μm and restricted volume fractions of 0.1-0.7. In experiments, the TractCaliber method accurately estimated restricted volume fractions within taxons in the parallel fiber portions of the phantom and showed decreased volume fraction estimates at the fiber crossings (Fig. 2B). The TractCaliber method overestimated taxon diameters by approximately 15-25% for volume fractions less than 80% and achieved estimates close to the actual 12μm inner diameter for the highest volume fraction of 80% (Fig. 2A). The taxon diameter estimates were fairly uniform throughout the phantom, including at fiber crossings.Discussion
We demonstrate experimental validation of taxon diameter and density estimates by TractCaliber MRI in a novel phantom that mimics the diffusion properties of white matter. The estimates of volume fraction are consistent with those obtained by NODDI in a separate validation study on a related hollow textile phantom (8). The design of the phantom strives to emulate the underlying axonal microstructure more closely than existing phantoms (1, 2) and provides the added advantages of long-term stability, efficient production and reproducibility (3). Future work will focus on studying the influence of smaller diameter fibers, fiber crossings, and separation of restricted and hindered water by filling with deuterium in forthcoming iterations of the phantom.Conclusion
The TractCaliber method was used to obtain plausible microstructural estimates from a novel biomimetic phantom. These phantoms may prove useful for testing microstructural models relevant to characterizing white matter and for scanner and protocol calibration.Acknowledgements
Grant support: NIH NIBIB P41EB015896, U01MH093765, R00EB015445; NINDS K23NS096056; US Army W911QY-15-C-0043, VA ECNC F1880.References
1. Fieremans E, De Deene Y, Delputte S, Ozdemir MS, Achten E, Lemahieu I. The design of anisotropic diffusion phantoms for the validation of diffusion weighted magnetic resonance imaging. Phys Med Biol. 2008;53(19):5405-19.
2. Hubbard PL, Zhou FL, Eichhorn SJ, Parker GJ. Biomimetic phantom for the validation of diffusion magnetic resonance imaging. Magn Reson Med. 2015;73(1):299-305.
3. Guise C, Fernandes MM, Pathak S, Schneider W, Fangueiro R. Hollow polypropylene yarns as a biomimetic brain phantom for the validation of High-Definition Fiber Tractography imaging. ACS Appl Mater Interfaces. 2016.
4. Setsompop K, Kimmlingen R, Eberlein E, Witzel T, Cohen-Adad J, McNab JA, et al. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. Neuroimage. 2013;80:220-33.
5. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-90.
6. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870-88.
7. Huang SY, Witzel T, Fan Q, McNab JA, Wald LL, Nummenmaa A. TractCaliber: Axon diameter estimation across white matter tracts in the in vivo human brain using 300 mT/m gradients. Proceedings of the 23rd Annual Meeting of the ISMRM., 2015. Toronto, Canada.
8. Pathak S, Fissell C, Okonkwo D, Schneider W. Providing Ground Truth Quantification of Anisotropic Diffusion MRI Imaging with a Hollow Textile Phantom, Proc ISMRM 2017 (submitted).
Figures